Insufficient endometrial thickness, diagnosed as thin endometrium with a maximum thickness ≤ 7 mm on ultrasound scan accompanied by a normal uterine cavity, is closely associated with pregnancy failure. The most common causes of thin endometrium arise from inappropriate endometrium repair after curettage and surgical separation of intrauterine adhesion accompanied by disrupted blood vessel distribution and sparse glands. At present, the cellular and molecular mechanisms of thin endometrium remain ambiguous, and therapeutic options for thin endometrium are limited and controversial due to its complex pathogenesis. Hence, unraveling the function of different cell types, the regulation of cell proliferation and the features of thin endometrium are urgently required to develop specific and effective therapies.
In a study published in Proc Natl Acad Sci U S A (https://www.pnas.org/content/119/8/e2115912119.long), Prof. HU Yali’s team from Department of Obstetrics and Gynecology, Affiliated Drum Tower Hospital, Medical School of Nanjing University, in collaboration with Prof. DAI Jianwu's group from the Institute of Genetics and Developmental biology of the Chinese Academy of Sciences and Prof. DENG Wenbo’s group from Medical School of Xiamen University deciphered the transcriptional heterogeneity of endometrial niche in human thin endometrium at single-cell resolution.
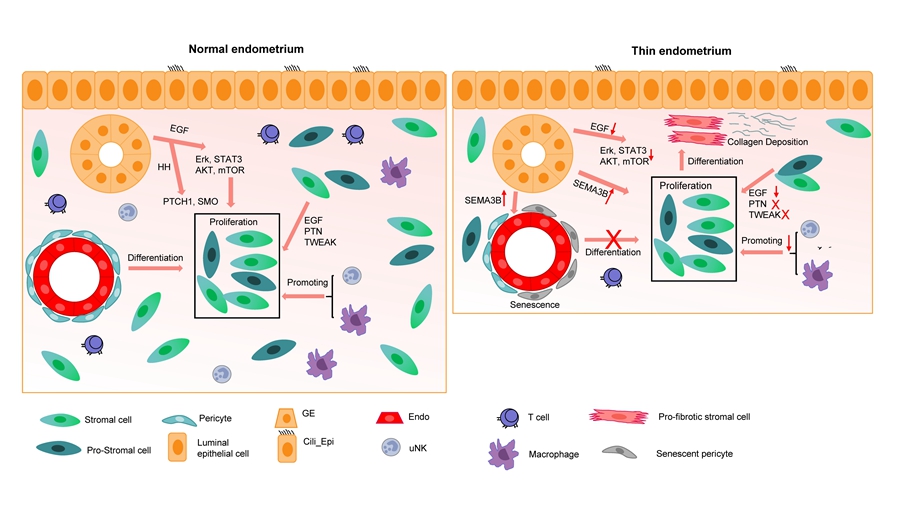
A schematic illustration showing cell type-specific genes involved in endometrial homeostasis in normal and thin endometrium. (Image by IGDB) The researchers profiled the transcriptomes of human endometrial cells by single-cell RNA sequencing and experimental verification to characterize cell types, their communications and the underlying mechanism of endometrial growth in normal and thin endometrium during the proliferative phase.
Stromal cells were the most abundant cell type in the endometrium, with a sub-population of proliferating stromal cells whose cell cycle signaling pathways were compromised in thin endometrium. The researchers also found that there was significantly up-regulated cellular senescence in the stroma and epithelium accompanied by collagen over-deposition around blood vessels. Moreover, decreased numbers of macrophages and NK cells further exacerbated the endometrial thinness. In addition, the over-activated SEMA3 and the dampened EGF, PTN and TWEAK signaling pathways were uncovered as causes for the insufficient proliferation of the endometrium and the deficient decidualization of stromal cells.
This study outlines the human endometrial cell composition during the proliferative phase at single-cell resolution in both normal and thin endometrium and described detailed cell type-specific gene signatures and communications between different cell types. The mechanistic insights arising from this study could establish new avenues for intervention development targeting ‘promoting proliferation of stromal cells’, ‘anti-senescence’ and ‘anti-collagen-overdeposition’ to protect against insufficient growth of the endometrium and related diseases and for developing new tools to rejuvenate the atrophic endometrium for female fertility preservation and successful pregnancy.
Contact:
Dr. DAI Jianwu
Institute of Genetics and Developmental biology, Chinese Academy of Sciences
 A schematic illustration showing cell type-specific genes involved in endometrial homeostasis in normal and thin endometrium. (Image by IGDB)The researchers profiled the transcriptomes of human endometrial cells by single-cell RNA sequencing and experimental verification to characterize cell types, their communications and the underlying mechanism of endometrial growth in normal and thin endometrium during the proliferative phase.Stromal cells were the most abundant cell type in the endometrium, with a sub-population of proliferating stromal cells whose cell cycle signaling pathways were compromised in thin endometrium. The researchers also found that there was significantly up-regulated cellular senescence in the stroma and epithelium accompanied by collagen over-deposition around blood vessels. Moreover, decreased numbers of macrophages and NK cells further exacerbated the endometrial thinness. In addition, the over-activated SEMA3 and the dampened EGF, PTN and TWEAK signaling pathways were uncovered as causes for the insufficient proliferation of the endometrium and the deficient decidualization of stromal cells.This study outlines the human endometrial cell composition during the proliferative phase at single-cell resolution in both normal and thin endometrium and described detailed cell type-specific gene signatures and communications between different cell types. The mechanistic insights arising from this study could establish new avenues for intervention development targeting ‘promoting proliferation of stromal cells’, ‘anti-senescence’ and ‘anti-collagen-overdeposition’ to protect against insufficient growth of the endometrium and related diseases and for developing new tools to rejuvenate the atrophic endometrium for female fertility preservation and successful pregnancy.Contact:Dr. DAI JianwuInstitute of Genetics and Developmental biology, Chinese Academy of SciencesEmail: jwdai@genetics.ac.cn
A schematic illustration showing cell type-specific genes involved in endometrial homeostasis in normal and thin endometrium. (Image by IGDB)The researchers profiled the transcriptomes of human endometrial cells by single-cell RNA sequencing and experimental verification to characterize cell types, their communications and the underlying mechanism of endometrial growth in normal and thin endometrium during the proliferative phase.Stromal cells were the most abundant cell type in the endometrium, with a sub-population of proliferating stromal cells whose cell cycle signaling pathways were compromised in thin endometrium. The researchers also found that there was significantly up-regulated cellular senescence in the stroma and epithelium accompanied by collagen over-deposition around blood vessels. Moreover, decreased numbers of macrophages and NK cells further exacerbated the endometrial thinness. In addition, the over-activated SEMA3 and the dampened EGF, PTN and TWEAK signaling pathways were uncovered as causes for the insufficient proliferation of the endometrium and the deficient decidualization of stromal cells.This study outlines the human endometrial cell composition during the proliferative phase at single-cell resolution in both normal and thin endometrium and described detailed cell type-specific gene signatures and communications between different cell types. The mechanistic insights arising from this study could establish new avenues for intervention development targeting ‘promoting proliferation of stromal cells’, ‘anti-senescence’ and ‘anti-collagen-overdeposition’ to protect against insufficient growth of the endometrium and related diseases and for developing new tools to rejuvenate the atrophic endometrium for female fertility preservation and successful pregnancy.Contact:Dr. DAI JianwuInstitute of Genetics and Developmental biology, Chinese Academy of SciencesEmail: jwdai@genetics.ac.cn CAS
CAS
 中文
中文




.png)
