Plants must maintain a delicate balance between gas exchange necessary for photosynthesis and water conservation in the surrounding environment, and they do this by regulating stomatal pores in the leaf epidermis. Stomatal pores act like tiny “mouths” to inhale CO2 and release O2. A great deal of water can evaporate through transpiration when these “mouths” are open. Plants can shut their “mouths” in response to environmental stimuli, such as drought, high-level CO2, ozone, and microbe, to prevent water loss, pollution, and pathogen invasion. The guard cells that form stomata can convert these signals into turgor pressure changes to regulate stomatal closure. It is well-established that guard cell anion effluxes are key events to trigger stomatal closure. Two distinct types of anion channels, SLAC1 (slow anion channel 1) and QUAC1 (fast anion channel 1), mediate the anion efflux from guard cells.
In 2021, CHEN Yu-hang’s group at the Institute of Genetics and Developmental Biology (IGDB), Chinese Academy of Sciences (CAS) solved the first cryo-EM structure of SLAC1(DOI: 10.1073/pnas.2015151118) and now has made another breakthrough in the study of QUAC1.
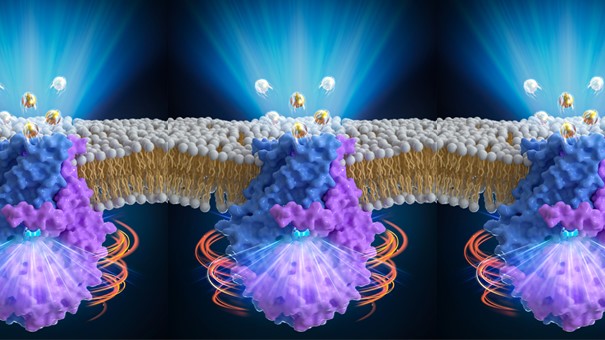
The structure and activation model of plant QUAC1/ALMT12 channel. A. The structure of QUAC1/ALMT12; B. The malate-mediated activation model. (Image by IGDB)
QUAC1, also known as ALMT12, belongs to the ALMT (Aluminum-activated Malate Transporters) family involved in various physiological processes, including stomatal function, pollen tube growth, aluminum resistance, mineral nutrition, fruit acidity, microbe interactions, cell signaling, and seed development. Among them, ALMT12 is involved in regulating stomatal closure, and is characterized as a rapid type (R-type) anion channel with quick activation/deactivation kinetics. The QUAC1/ALMT2 channels are pore-forming proteins embedded in the membrane, and they become unstable after extraction from the membrane. Researchers in Yu-hang Chen’s group screened for suitable detergents to extract and solubilize those molecules into solution, and determined their atomic structure by single-particle cryo-EM.
The overall molecule is a flat vase-shaped homodimer, forming a single T-shaped pore with a bifurcated entrance in the cytoplasm. The pore is lined with highly conserved positively charged residues, making its surface electro-positive for anions to pass through. The transmembrane domain (TMD) and the cytoplasmic helical domain (CHD) interact in a high-energy twisted manner, providing the basis for rapid gating regulation. Using a two-electrode voltage-clamp technique, scientists found that QUAC1/ALMT2-mediated currents display rapid activation/deactivation kinetics and strong voltage dependency, a hallmark feature of R-type anion current in guard cells. In the planar lipid bilayer experiments, scientists found that malate stimulates channel activity via increasing open probability. Altogether, structural analysis accompanied with electrophysiological studies provided insights into the gating and activity regulation of the novel QUAC1/ALMT12 channel. These findings lay the foundation for further research on the molecular mechanism of stomatal regulation and provide important information for designing drought-resistant and water-saving crops.
This work, entitled "Cryo-EM structure and electrophysiological characterization of ALMT from
Glycine max reveal a previously uncharacterized class of anion channels" (
DOI: 10.1126/sciadv.abm3238), was published in
Science Advances March 2, 2022.
This work was jointly conducted by Dr ZHAI Yujia’s team and Dr. SUN Fei’s team from the Institute of Biophysics of CAS. It was funded by the National Key R&D Program of China, the National Natural Science Foundation of China, and the Strategic Priority Research Program of CAS.
Contact
Dr. CHEN Yu-hang
Institute of Genetics and Developmental Biology, Chinese Academy of Sciences
 The structure and activation model of plant QUAC1/ALMT12 channel. A. The structure of QUAC1/ALMT12; B. The malate-mediated activation model. (Image by IGDB)QUAC1, also known as ALMT12, belongs to the ALMT (Aluminum-activated Malate Transporters) family involved in various physiological processes, including stomatal function, pollen tube growth, aluminum resistance, mineral nutrition, fruit acidity, microbe interactions, cell signaling, and seed development. Among them, ALMT12 is involved in regulating stomatal closure, and is characterized as a rapid type (R-type) anion channel with quick activation/deactivation kinetics. The QUAC1/ALMT2 channels are pore-forming proteins embedded in the membrane, and they become unstable after extraction from the membrane. Researchers in Yu-hang Chen’s group screened for suitable detergents to extract and solubilize those molecules into solution, and determined their atomic structure by single-particle cryo-EM.The overall molecule is a flat vase-shaped homodimer, forming a single T-shaped pore with a bifurcated entrance in the cytoplasm. The pore is lined with highly conserved positively charged residues, making its surface electro-positive for anions to pass through. The transmembrane domain (TMD) and the cytoplasmic helical domain (CHD) interact in a high-energy twisted manner, providing the basis for rapid gating regulation. Using a two-electrode voltage-clamp technique, scientists found that QUAC1/ALMT2-mediated currents display rapid activation/deactivation kinetics and strong voltage dependency, a hallmark feature of R-type anion current in guard cells. In the planar lipid bilayer experiments, scientists found that malate stimulates channel activity via increasing open probability. Altogether, structural analysis accompanied with electrophysiological studies provided insights into the gating and activity regulation of the novel QUAC1/ALMT12 channel. These findings lay the foundation for further research on the molecular mechanism of stomatal regulation and provide important information for designing drought-resistant and water-saving crops.This work, entitled "Cryo-EM structure and electrophysiological characterization of ALMT from Glycine max reveal a previously uncharacterized class of anion channels" (DOI: 10.1126/sciadv.abm3238), was published in Science Advances March 2, 2022.This work was jointly conducted by Dr ZHAI Yujia’s team and Dr. SUN Fei’s team from the Institute of Biophysics of CAS. It was funded by the National Key R&D Program of China, the National Natural Science Foundation of China, and the Strategic Priority Research Program of CAS.ContactDr. CHEN Yu-hangInstitute of Genetics and Developmental Biology, Chinese Academy of SciencesE-mail: yuhang.chen@genetics.ac.cn
The structure and activation model of plant QUAC1/ALMT12 channel. A. The structure of QUAC1/ALMT12; B. The malate-mediated activation model. (Image by IGDB)QUAC1, also known as ALMT12, belongs to the ALMT (Aluminum-activated Malate Transporters) family involved in various physiological processes, including stomatal function, pollen tube growth, aluminum resistance, mineral nutrition, fruit acidity, microbe interactions, cell signaling, and seed development. Among them, ALMT12 is involved in regulating stomatal closure, and is characterized as a rapid type (R-type) anion channel with quick activation/deactivation kinetics. The QUAC1/ALMT2 channels are pore-forming proteins embedded in the membrane, and they become unstable after extraction from the membrane. Researchers in Yu-hang Chen’s group screened for suitable detergents to extract and solubilize those molecules into solution, and determined their atomic structure by single-particle cryo-EM.The overall molecule is a flat vase-shaped homodimer, forming a single T-shaped pore with a bifurcated entrance in the cytoplasm. The pore is lined with highly conserved positively charged residues, making its surface electro-positive for anions to pass through. The transmembrane domain (TMD) and the cytoplasmic helical domain (CHD) interact in a high-energy twisted manner, providing the basis for rapid gating regulation. Using a two-electrode voltage-clamp technique, scientists found that QUAC1/ALMT2-mediated currents display rapid activation/deactivation kinetics and strong voltage dependency, a hallmark feature of R-type anion current in guard cells. In the planar lipid bilayer experiments, scientists found that malate stimulates channel activity via increasing open probability. Altogether, structural analysis accompanied with electrophysiological studies provided insights into the gating and activity regulation of the novel QUAC1/ALMT12 channel. These findings lay the foundation for further research on the molecular mechanism of stomatal regulation and provide important information for designing drought-resistant and water-saving crops.This work, entitled "Cryo-EM structure and electrophysiological characterization of ALMT from Glycine max reveal a previously uncharacterized class of anion channels" (DOI: 10.1126/sciadv.abm3238), was published in Science Advances March 2, 2022.This work was jointly conducted by Dr ZHAI Yujia’s team and Dr. SUN Fei’s team from the Institute of Biophysics of CAS. It was funded by the National Key R&D Program of China, the National Natural Science Foundation of China, and the Strategic Priority Research Program of CAS.ContactDr. CHEN Yu-hangInstitute of Genetics and Developmental Biology, Chinese Academy of SciencesE-mail: yuhang.chen@genetics.ac.cn CAS
CAS
 中文
中文




.png)
