Fusarium graminearum is a widespread pathogenic fungus that causes Fusarium head blight (FHB) in cereal crops worldwide, especially in wheat. Between 2000 and 2018, more than 4.5 million hectares were annually affected by FHB in China, or around 20% of the total planted area of wheat. This has resulted in annual production losses of more than 3.41 million tons.
Wheat heads with Fusarium head blight symptoms on a few spikelets (left-hand panel), and the fungal fruiting body on a stubble (right-hand panel) (Image by IGDB).
Recently, researchers in Prof. BAI Yang’s lab from the Institute of Genetics and Developmental Biology, Chinese Academy of Sciences, Prof. CHEN Yun’s lab and Prof. YU Yunlong’s lab at Zhejiang University, found that F. graminearum perithecia provided a specific ecological niche for bacteria that could play an important role in disease establishment.
Over 2000 bacterial strains were isolated from the native microbiota of F. graminearum, and subsequently identified and screened for antagonistic activity. They were successful in this approach and recovered 113 isolates that showed antagonistic activity against the pathogen. One specific bacterial isolate, termed as ZJU23, was identified as Pantoea agglomerans, and had the strongest inhibitory ability against F. graminearum among all isolates. Subsequently, they found that herbicolin A secreted by ZJU23 is responsible for the observed suppression of F. graminearum.
Although herbicolin A was identified about four decades ago, its biosynthetic gene cluster and mode of action against fungi were not known. In this study, they set out to investigate the biosynthetic gene cluster and mode of action against various fungi by combining various approaches, including transposon mutagenesis, liquid chromatography-mass spectrometry, atomic force microscopy and confocal microscopy.
By comparing the metabolic profiles of ZJU23 and four deletion mutants of a potential biosynthesis gene cluster, they found that herbicolin A was synthesized by the AcbA-AcbJ cluster. They then proceeded to uncover the mode of action of herbicolin A against various fungi.
It is important to note that the modes of action of cyclic lipopeptides against fungi are mostly unknown. It’s surprise that herbicolin A was shown to disrupt lipid rafts by interacting with ergosterol, which resulted in the formation of abnormal cell membranes, and, ultimately, caused cell death. Herbicolin A was also found to inhibit the growth of Candida albicans and Aspergillus fumigatus, and was more effective than the clinical fungicides Amphotericin B and fluconazole. This provided important evidence for potential applications of herbicolin A beyond agriculture, for example in medicine.
Overall, the reserchers have deciphered the mechanism of the inhibitory effect of herbicolin A on fungi and identified its biosynthetic gene cluster. This could support future developments for sustainable management of Fusarium head blight worldwide.
Proposed model for the mode of action of herbicolin A secreted by ZJU23 in the fungal perithecium microbiome.(Image by IGDB)
This work, entitled “Fusarium fruiting body microbiome member
Pantoea agglomerans inhibits fungal pathogenesis by targeting lipid rafts”, was published in
Nature Microbiology (
https://doi.org/10.1038/s41564-022-01131-x).
Contact:
Prof. BAI Yang
Institute of Genetics and Developmental Biology, Chinese Academy of Sciences
 Wheat heads with Fusarium head blight symptoms on a few spikelets (left-hand panel), and the fungal fruiting body on a stubble (right-hand panel) (Image by IGDB).Recently, researchers in Prof. BAI Yang’s lab from the Institute of Genetics and Developmental Biology, Chinese Academy of Sciences, Prof. CHEN Yun’s lab and Prof. YU Yunlong’s lab at Zhejiang University, found that F. graminearum perithecia provided a specific ecological niche for bacteria that could play an important role in disease establishment.Over 2000 bacterial strains were isolated from the native microbiota of F. graminearum, and subsequently identified and screened for antagonistic activity. They were successful in this approach and recovered 113 isolates that showed antagonistic activity against the pathogen. One specific bacterial isolate, termed as ZJU23, was identified as Pantoea agglomerans, and had the strongest inhibitory ability against F. graminearum among all isolates. Subsequently, they found that herbicolin A secreted by ZJU23 is responsible for the observed suppression of F. graminearum.Although herbicolin A was identified about four decades ago, its biosynthetic gene cluster and mode of action against fungi were not known. In this study, they set out to investigate the biosynthetic gene cluster and mode of action against various fungi by combining various approaches, including transposon mutagenesis, liquid chromatography-mass spectrometry, atomic force microscopy and confocal microscopy.By comparing the metabolic profiles of ZJU23 and four deletion mutants of a potential biosynthesis gene cluster, they found that herbicolin A was synthesized by the AcbA-AcbJ cluster. They then proceeded to uncover the mode of action of herbicolin A against various fungi.It is important to note that the modes of action of cyclic lipopeptides against fungi are mostly unknown. It’s surprise that herbicolin A was shown to disrupt lipid rafts by interacting with ergosterol, which resulted in the formation of abnormal cell membranes, and, ultimately, caused cell death. Herbicolin A was also found to inhibit the growth of Candida albicans and Aspergillus fumigatus, and was more effective than the clinical fungicides Amphotericin B and fluconazole. This provided important evidence for potential applications of herbicolin A beyond agriculture, for example in medicine.Overall, the reserchers have deciphered the mechanism of the inhibitory effect of herbicolin A on fungi and identified its biosynthetic gene cluster. This could support future developments for sustainable management of Fusarium head blight worldwide.
Wheat heads with Fusarium head blight symptoms on a few spikelets (left-hand panel), and the fungal fruiting body on a stubble (right-hand panel) (Image by IGDB).Recently, researchers in Prof. BAI Yang’s lab from the Institute of Genetics and Developmental Biology, Chinese Academy of Sciences, Prof. CHEN Yun’s lab and Prof. YU Yunlong’s lab at Zhejiang University, found that F. graminearum perithecia provided a specific ecological niche for bacteria that could play an important role in disease establishment.Over 2000 bacterial strains were isolated from the native microbiota of F. graminearum, and subsequently identified and screened for antagonistic activity. They were successful in this approach and recovered 113 isolates that showed antagonistic activity against the pathogen. One specific bacterial isolate, termed as ZJU23, was identified as Pantoea agglomerans, and had the strongest inhibitory ability against F. graminearum among all isolates. Subsequently, they found that herbicolin A secreted by ZJU23 is responsible for the observed suppression of F. graminearum.Although herbicolin A was identified about four decades ago, its biosynthetic gene cluster and mode of action against fungi were not known. In this study, they set out to investigate the biosynthetic gene cluster and mode of action against various fungi by combining various approaches, including transposon mutagenesis, liquid chromatography-mass spectrometry, atomic force microscopy and confocal microscopy.By comparing the metabolic profiles of ZJU23 and four deletion mutants of a potential biosynthesis gene cluster, they found that herbicolin A was synthesized by the AcbA-AcbJ cluster. They then proceeded to uncover the mode of action of herbicolin A against various fungi.It is important to note that the modes of action of cyclic lipopeptides against fungi are mostly unknown. It’s surprise that herbicolin A was shown to disrupt lipid rafts by interacting with ergosterol, which resulted in the formation of abnormal cell membranes, and, ultimately, caused cell death. Herbicolin A was also found to inhibit the growth of Candida albicans and Aspergillus fumigatus, and was more effective than the clinical fungicides Amphotericin B and fluconazole. This provided important evidence for potential applications of herbicolin A beyond agriculture, for example in medicine.Overall, the reserchers have deciphered the mechanism of the inhibitory effect of herbicolin A on fungi and identified its biosynthetic gene cluster. This could support future developments for sustainable management of Fusarium head blight worldwide.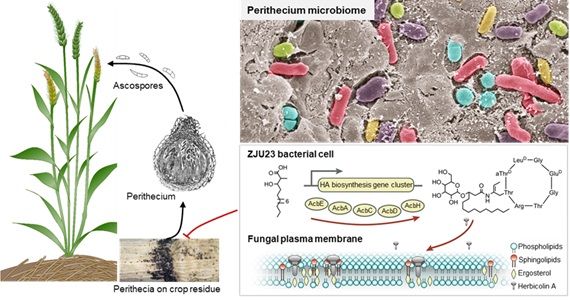 Proposed model for the mode of action of herbicolin A secreted by ZJU23 in the fungal perithecium microbiome.(Image by IGDB)This work, entitled “Fusarium fruiting body microbiome member Pantoea agglomerans inhibits fungal pathogenesis by targeting lipid rafts”, was published in Nature Microbiology (https://doi.org/10.1038/s41564-022-01131-x).Contact:Prof. BAI YangInstitute of Genetics and Developmental Biology, Chinese Academy of SciencesEmail: byang@genetics.ac.cn
Proposed model for the mode of action of herbicolin A secreted by ZJU23 in the fungal perithecium microbiome.(Image by IGDB)This work, entitled “Fusarium fruiting body microbiome member Pantoea agglomerans inhibits fungal pathogenesis by targeting lipid rafts”, was published in Nature Microbiology (https://doi.org/10.1038/s41564-022-01131-x).Contact:Prof. BAI YangInstitute of Genetics and Developmental Biology, Chinese Academy of SciencesEmail: byang@genetics.ac.cn CAS
CAS
 中文
中文




.png)
