Plants possess a sophisticated innate immune surveillance system to recognize microbial molecules and protect themselves against invading pathogens. The plasma membrane-localized immune receptors sense conserved Pathogen-Associate
d Molecular Patterns (PAMPs) to activate PAMP-triggered immunity (PTI), while intracellular resistance(R) proteins recognize pathogen effector proteins inside the plant cell to initiate effector-triggered immunity (ETI). Phytopathogenic bacteria secrete a number of effector proteins into the host cell to suppress host immunity, promoting parasitism. However, biochemical function and molecular basis for the vast majority of effectors are largely unknown.
Xanthomonas campestrispv
campestris (
Xcc) is a causal agent of black rot diseases on numerous crucifer plants such as
Brassica and Arabidopsis. The type III effector protein AvrAC exists in all sequenced strains of
Xcc. Using the Arabidopsis-
Xcc as a model system, Jian-Min Zhou’s group at the Institute of Genetics and Developmental Biology, the Chinese Academy of Sciences demonstrate that AvrAC can strongly inhibit plant PTI and ETI and contribute to
Xcc virulence in Arabidopsis by specifically targeting Arabidopsis BIK1 and RIPK, two receptor-like cytoplasmic kinases (RLCKs) known to mediate immune signaling. Molecular and biochemical analyses revealed that AvrAC is an uridylyl transferase that uridylylates the conserved serine and threonine residues in the activation loop of BIK1 and RIPK. The uridylylation on these residues masks the
phosphorylation sites, inhibits the kinase activity, and blocks downstream signaling. AvrAC is the first effector protein known to possess uridylyl transferase activity.
The workillustrates a unique biochemical mechanism by which the
Xcc bacterium combats the plant innate immune system.
This work was published online in Nature on April 15 (Doi:10.1038/nature10962). Graduate student Feng Feng is the first author of the paper. Dr. Chaozu He at
Hainan University is a co-corresponding author of the paper. This research was supported by grants from the Chinese Ministry of Science and Technology.
AUTHOR CONTACT:
Jian-Min Zhou, Ph.D.
Institute of Genetics and Developmetnal Biology,
Chinese Academy of Sciences, Beijing, China.
(Image by Feng Feng etc.)
Figure. AvrAC uridylylates plant immune kinases to inhibit immune signaling.
(A). Tandem mass (MS/MS) spectrometric analysis of uridylylation of conserved serine and threonine residues in the activation loop of BIK1 by AvrAC in plant cell. (B). AvrAC contributes to Xcc virulence in Arabidopsis plants in a BIK1-dependent manner. Wide type Xcc and avrAC mutant strains were co-infiltrated into Arabidopsis plants of the indicated geneotypes for competitive index assay. (C). A model for AvrAC-catalyzed uridylylation and inhibition of plant immunity. In normal plants (left), BIK1 and RIPK are phosphorylated upon the stimulation by upstream immune receptor kinase complexes or effector AvrB. In plants that are infected with Xcc (right), AvrAC uridylylates the conserved serine and threonine residues in the activation loop of BIK1 and RIPK, thereby preventing the phosphorylation-dependent activation of these kinases and downstream signaling.
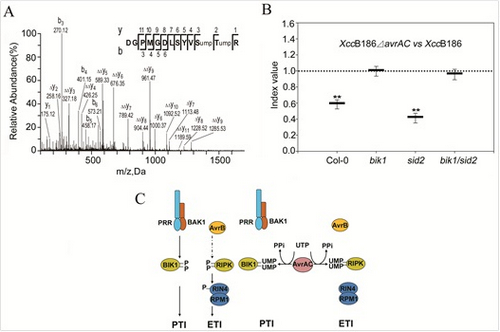 (Image by Feng Feng etc.)Figure. AvrAC uridylylates plant immune kinases to inhibit immune signaling.(A). Tandem mass (MS/MS) spectrometric analysis of uridylylation of conserved serine and threonine residues in the activation loop of BIK1 by AvrAC in plant cell. (B). AvrAC contributes to Xcc virulence in Arabidopsis plants in a BIK1-dependent manner. Wide type Xcc and avrAC mutant strains were co-infiltrated into Arabidopsis plants of the indicated geneotypes for competitive index assay. (C). A model for AvrAC-catalyzed uridylylation and inhibition of plant immunity. In normal plants (left), BIK1 and RIPK are phosphorylated upon the stimulation by upstream immune receptor kinase complexes or effector AvrB. In plants that are infected with Xcc (right), AvrAC uridylylates the conserved serine and threonine residues in the activation loop of BIK1 and RIPK, thereby preventing the phosphorylation-dependent activation of these kinases and downstream signaling.
(Image by Feng Feng etc.)Figure. AvrAC uridylylates plant immune kinases to inhibit immune signaling.(A). Tandem mass (MS/MS) spectrometric analysis of uridylylation of conserved serine and threonine residues in the activation loop of BIK1 by AvrAC in plant cell. (B). AvrAC contributes to Xcc virulence in Arabidopsis plants in a BIK1-dependent manner. Wide type Xcc and avrAC mutant strains were co-infiltrated into Arabidopsis plants of the indicated geneotypes for competitive index assay. (C). A model for AvrAC-catalyzed uridylylation and inhibition of plant immunity. In normal plants (left), BIK1 and RIPK are phosphorylated upon the stimulation by upstream immune receptor kinase complexes or effector AvrB. In plants that are infected with Xcc (right), AvrAC uridylylates the conserved serine and threonine residues in the activation loop of BIK1 and RIPK, thereby preventing the phosphorylation-dependent activation of these kinases and downstream signaling. CAS
CAS
 中文
中文




.png)
