Both animal and plant innate immune systems relies on detection of conserved signature components of pathogens, termed Pathogen-Associated Molecular Patterns (PAMPs), for stimulating anti-microbial responses. In plants, perception of PAMPs is mainly mediated by the membrane-localized pattern recognition receptors (PRRs). Upon recognition of PAMPs, PRRs initiate an array of shared immune responses (PAMP-trigged immunity, PTI)
FLS2 is an LRR-RK and acts as the PRR for bacterial flagellin by recognizing flg22. Direct recognition of flg22 by FLS2 is sufficient for inducing immune responses, establishing FLS2 as a flagellin receptor. Flg22 binding nearly simultaneously triggers FLS2 association with the LRR-RK BAK1, a positive regulator of brassinosteroid (BR) signaling that interacts with the LRR-RK BR INSENSITIVE 1 (BRI1).
To reveal the molecular mechanisms underlying flg22-induced activation of FLS2, Scientists in Jianmin Zhou’s lab from the Institute of Genetics and Developmental Biology collaborated with scientists in Jijie Chai’s lab from Tsinghua university report the crystal structure of flg22 in complex with the ectodomains of FLS2 and BAK1. A conserved and a non-conserved site from the inner surface of the FLS2 solenoid recognize the C- and N-terminal side of flg22 respectively, involving no oligomerization and conformational changes in FLS2. BAK1 acts as a secondary receptor by recognizing the C-terminus of the FLS2-bound flg22. Consistently, mutations compromising the FLS2-BAK1 interactions attenuate the flg22-induced activation of FLS2 and immune responses.
FLS2LRR-flg22 -BAK1LRR is the first LRR complex structure in plant to be revealed. Arabidopsis contains more than 200 LRRs receptor kinases (there are about 600 in the rice). LRR-RLKs involved in a variety of biological processes, including the growth of the meristem, disease resistance, hormone signaling, and organization development. The results provide a good example for research about this kind of proteins structure and function. Besides, the research results will help the design of broad-spectrum resistance crops.
This work with Yadong Sun and Lei Li as the first coauthor has been published on Science (http://www.sciencemag.org/content/342/6158/624). This research was supported by grants from Chinese Natural Science Foundation and Chinese Ministry of Science and Technology.
AUTHOR CONTACT:
Jian-min Zhou, Principal Investigator.
Institute of Genetics and Developmetnal Biology, Chinese Academy of Sciences, Beijing, China.
E-mail: jmzhou@genetics.ac.cn
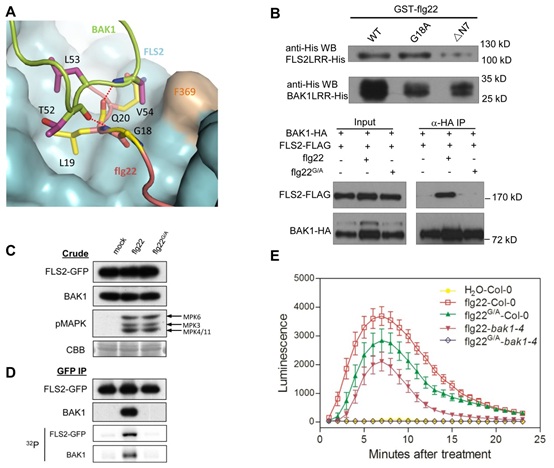
Figure. BAK1 recognizes the C-terminal side of the FLS2-bound flg22
(A) A selective role of flg22 Gly18 in interaction with BAK1. FLS2LRR is shown in surface (blue), with flg22 (salmon) and BAK1LRR (green). The side chains from BAK1LRR and flg22 are shown in purple and yellow, respectively. (B) Gly18 is required for the flg22-induced FLS2LRR-BAK1LRR interaction but
not for FLS2 binding in vitro and in null fls2 mutant mesophyll protoplasts. (C) The Flg22 G18A mutation has little effect on MPK phosphorylation in mesophyll protoplasts.(D) Gly18 is required for flg22-induced FLS2-BAK1 interaction and the complex activation in seedlings. GFP, green fluorescent protein. (E) The flg22 G18A mutation modestly affects generation of ROS in planta. WT Arabidopsis leaves were treated with water, 100 nM flg22, or 100 nM flg22 G18A.
 Figure. BAK1 recognizes the C-terminal side of the FLS2-bound flg22(A) A selective role of flg22 Gly18 in interaction with BAK1. FLS2LRR is shown in surface (blue), with flg22 (salmon) and BAK1LRR (green). The side chains from BAK1LRR and flg22 are shown in purple and yellow, respectively. (B) Gly18 is required for the flg22-induced FLS2LRR-BAK1LRR interaction butnot for FLS2 binding in vitro and in null fls2 mutant mesophyll protoplasts. (C) The Flg22 G18A mutation has little effect on MPK phosphorylation in mesophyll protoplasts.(D) Gly18 is required for flg22-induced FLS2-BAK1 interaction and the complex activation in seedlings. GFP, green fluorescent protein. (E) The flg22 G18A mutation modestly affects generation of ROS in planta. WT Arabidopsis leaves were treated with water, 100 nM flg22, or 100 nM flg22 G18A.
Figure. BAK1 recognizes the C-terminal side of the FLS2-bound flg22(A) A selective role of flg22 Gly18 in interaction with BAK1. FLS2LRR is shown in surface (blue), with flg22 (salmon) and BAK1LRR (green). The side chains from BAK1LRR and flg22 are shown in purple and yellow, respectively. (B) Gly18 is required for the flg22-induced FLS2LRR-BAK1LRR interaction butnot for FLS2 binding in vitro and in null fls2 mutant mesophyll protoplasts. (C) The Flg22 G18A mutation has little effect on MPK phosphorylation in mesophyll protoplasts.(D) Gly18 is required for flg22-induced FLS2-BAK1 interaction and the complex activation in seedlings. GFP, green fluorescent protein. (E) The flg22 G18A mutation modestly affects generation of ROS in planta. WT Arabidopsis leaves were treated with water, 100 nM flg22, or 100 nM flg22 G18A. CAS
CAS
 中文
中文




.png)
