Synapses are highly specialized intercellular junctions that transmit information between neurons and their targets. Studies on the formation and development of synapses will shed light not only on the physiological neurodevelopment but also the pathogenesis of related neurological diseases. The S6 kinase (S6K) is a protein kinase involved in multiple cellular and developmental processes. But its function in neuronal system is not well understood.
The group of Prof. ZHANG Yongqing from the Institute of Genetics and Developmental Biology, Chinese Academy of Sciences, studies the mechanism of synapse development and function, as well as pathogenesis of neurological diseases. From a genetic screen for genes that regulate synapse development, they unraveled that one member of the S6K family, they named S6K-like (S6KL), restrains Drosophila neuromuscular junction (NMJ) growth by inhibiting bone morphogenetic protein (BMP) signaling activity. This protein is evolutionarily conserved and has not been identified in any organism before.
S6KL mutants were fully adult viable but displayed distinct NMJ morphological and function abnormalities, including more synaptic boutons, especially more satellite boutons, fewer and larger synaptic vesicles, reduced synaptic endocytosis and increased amplitudes of miniature excitatory junctional potential (mEJP). Genetic analysis showed that reducing the dose of thickveins, encoding a type I receptor of BMP ligands, by half in S6KL null background rescued the NMJ overgrowth phenotype. Furthermore, the bouton number was significantly increased in dad, encoding a negative regulator of BMP signaling, and S6KL trans-heterozygotes compared to the wild type.
Finally, the level of BMP receptor thickveins (Tkv) was increased in S6KL mutants compared with the wild type. Biochemically, Tkv physically interacted with S6KL in S2 cells. Furthermore, Tkv protein level was increased in S6KL knockdown and decreased in S6KL overexpressing S2 cells. The reduction of the Tkv protein level which caused by S6KL overexpression could be blocked using the proteasome inhibitor MG132, suggesting that S6KL promotes Tkv degradation through proteosome degradation pathways. Together, these results demonstrate that S6KL regulates synaptic development via promoting BMP type I receptor Tkv degradation.
This is the first report of a critical role for S6KL at Drosophila NMJs by inhibiting BMP signaling activity. As S6KL is highly conserved from C. elegans to human and has not been studied before, the findings from this study will shed light on S6KL’s function in mammals.
This work has been published in PLoS Genetics on March 6, 2015 (DOI:10.1371/journal.pgen.1004984), with Doctor ZHAO Guoli as the first author. This work was supported by the National Science Foundation of China, the Chinese Academy of Sciences and the Ministry of Science and Technology.
Figure. Genetic interactions between S6KL and components of the BMP signaling pathway in regulating NMJ growth.
(A–D) Confocal images of NMJ 4 synapses of different genotypes co-stained with anti-HRP (green) and anti-CSP (magenta): WT (A), S6KL140 (B), S6KL140; tkv7/+ (C) and tkv7/tkvk16713 (D). Scale bar, 5 μm. (E–H) Confocal images of NMJ 4 terminals co-labeled with anti-pMad (green) and anti-HRP (magenta) in WT (E), tkv7/+ (F), S6KL140 (G), and S6KL140; tkv7/+ (H) larvae. Scale bar, 5 μm. (I) A model for the function of S6KL in regulation of BMP signaling at NMJs. S6KL inhibits BMP receptor Tkv protein level by promoting Tkv degradation, ultimately inhibits BMP signaling activity and NML growth. (Image by IGDB)
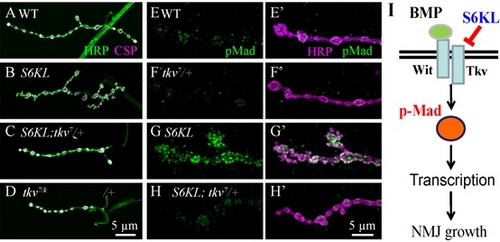 Figure. Genetic interactions between S6KL and components of the BMP signaling pathway in regulating NMJ growth.(A–D) Confocal images of NMJ 4 synapses of different genotypes co-stained with anti-HRP (green) and anti-CSP (magenta): WT (A), S6KL140 (B), S6KL140; tkv7/+ (C) and tkv7/tkvk16713 (D). Scale bar, 5 μm. (E–H) Confocal images of NMJ 4 terminals co-labeled with anti-pMad (green) and anti-HRP (magenta) in WT (E), tkv7/+ (F), S6KL140 (G), and S6KL140; tkv7/+ (H) larvae. Scale bar, 5 μm. (I) A model for the function of S6KL in regulation of BMP signaling at NMJs. S6KL inhibits BMP receptor Tkv protein level by promoting Tkv degradation, ultimately inhibits BMP signaling activity and NML growth. (Image by IGDB)
Figure. Genetic interactions between S6KL and components of the BMP signaling pathway in regulating NMJ growth.(A–D) Confocal images of NMJ 4 synapses of different genotypes co-stained with anti-HRP (green) and anti-CSP (magenta): WT (A), S6KL140 (B), S6KL140; tkv7/+ (C) and tkv7/tkvk16713 (D). Scale bar, 5 μm. (E–H) Confocal images of NMJ 4 terminals co-labeled with anti-pMad (green) and anti-HRP (magenta) in WT (E), tkv7/+ (F), S6KL140 (G), and S6KL140; tkv7/+ (H) larvae. Scale bar, 5 μm. (I) A model for the function of S6KL in regulation of BMP signaling at NMJs. S6KL inhibits BMP receptor Tkv protein level by promoting Tkv degradation, ultimately inhibits BMP signaling activity and NML growth. (Image by IGDB) CAS
CAS
 中文
中文




.png)
