Huntington’s disease (HD) is caused by polyglutamine (polyQ) expansion in the N-terminal region of huntingtin (HTT). Despite its ubiquitous expression in the brain and body, mutant HTT causes selective neuronal degeneration as well as white matter atrophy in the brain. Previous reports show that reducing the expression of mutant HTT is an important strategy to alleviate HD pathology and phenotypes in HD mice. Thus, considerable efforts have been devoted to developing siRNA and anti-sense oligonucleotides to suppress the expression of mutant HTT in adult brains. Unfortunately, however, these approaches have also raised concerns that markedly suppressing HTT expression will lead to side effects by impairing HTT’s normal function.
In a related article, a team led by Dr. LI Xiaojiang of the State Key Laboratory of Developmental Biology, Institute of Genetics and Developmental Biology (IGDB), Chinese Academy of Sciences, explored the elusive function of huntingtin in adult tissues, in light of ongoing therapeutic attempts to hobble mutant huntingtin using genetic techniques in the adult mouse brain.
The researchers reported that depleting huntingtin in adult mouse neurons did not trigger Huntington's-related neurodegeneration or discernible symptoms. However, loss of huntingtin in 2-month-old mice proved lethal, likely due to acute pancreatitis, marked by the loss of pancreatic acinar cells, which undergo degeneration mediated by the digestive enzyme trypsin that is normally kept in check by huntingtin. Expressing a truncated huntingtin protein lacking the N-terminal region in huntingtin-depleted mice ameliorated pancreatitis and prevented death.
According to the studies huntingtin might thus play age- and cell type-specific roles in humans, and the findings point to potential time-sensitive therapeutic gateways for the disease. These studies suggest that the pathogenesis of HD is due to a gain of toxicity of mutant Htt and that depletion of Htt in adults could be an effective therapy.
The work entitled “Ablation of huntingtin in adult neurons is non-deleterious but its depletion in young mice causes acute pancreatitis” was published in
Proc Natl Acad Sci USA on March 7, 2016 (
DOI:10.1073/pnas.1524575113).
This research was supported by the grants from National Natural Science Foundation, Strategic Priority Research Program of the Chinese Academy of Sciences, and the State Key Laboratory of Molecular Developmental Biology, China.
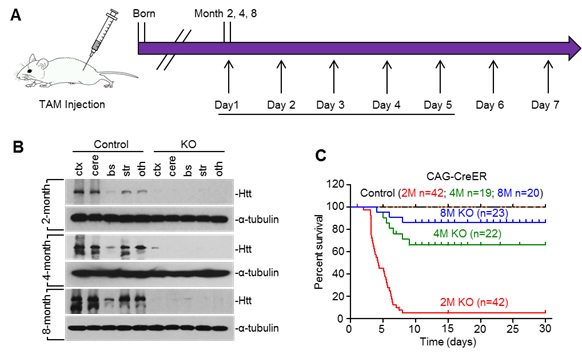
Loss of Htt-mediated age-dependent death in mice. Control is heterozygous floxed Htt/CAG-CreER mice injected with tamoxifen, and KO is homozygous floxed Htt/CAG-CreER mice injected with tamoxifen. (A) Diagram depicting inactivation of the Htt gene in adult mouse expressing CreER. The mice were intraperitoneally (I.P.) injected with tamoxifen (TAM) 20 mg/ml for 5 continuous days; Mice were examined for 7-11 months after tamoxifen injection. (B) Western blots showing relative Htt protein levels in the brain of ubiquitous KO and control mice at 2, 4, and 8 months of age. Ctx: cortex; cere: cerebellum; bs: brain stem; str: striatum; oth: other brain regions. (C) Survival rate of ubiquitous KO mouse and control mice when they were injected with tamoxifen at 2, 4, and 8 months of age. (Image by WANG et al.)
Contact:
Dr. LI Xiaojiang
E-mail: xjli@genetics.ac.cn
 Loss of Htt-mediated age-dependent death in mice. Control is heterozygous floxed Htt/CAG-CreER mice injected with tamoxifen, and KO is homozygous floxed Htt/CAG-CreER mice injected with tamoxifen. (A) Diagram depicting inactivation of the Htt gene in adult mouse expressing CreER. The mice were intraperitoneally (I.P.) injected with tamoxifen (TAM) 20 mg/ml for 5 continuous days; Mice were examined for 7-11 months after tamoxifen injection. (B) Western blots showing relative Htt protein levels in the brain of ubiquitous KO and control mice at 2, 4, and 8 months of age. Ctx: cortex; cere: cerebellum; bs: brain stem; str: striatum; oth: other brain regions. (C) Survival rate of ubiquitous KO mouse and control mice when they were injected with tamoxifen at 2, 4, and 8 months of age. (Image by WANG et al.)Contact:Dr. LI XiaojiangE-mail: xjli@genetics.ac.cn
Loss of Htt-mediated age-dependent death in mice. Control is heterozygous floxed Htt/CAG-CreER mice injected with tamoxifen, and KO is homozygous floxed Htt/CAG-CreER mice injected with tamoxifen. (A) Diagram depicting inactivation of the Htt gene in adult mouse expressing CreER. The mice were intraperitoneally (I.P.) injected with tamoxifen (TAM) 20 mg/ml for 5 continuous days; Mice were examined for 7-11 months after tamoxifen injection. (B) Western blots showing relative Htt protein levels in the brain of ubiquitous KO and control mice at 2, 4, and 8 months of age. Ctx: cortex; cere: cerebellum; bs: brain stem; str: striatum; oth: other brain regions. (C) Survival rate of ubiquitous KO mouse and control mice when they were injected with tamoxifen at 2, 4, and 8 months of age. (Image by WANG et al.)Contact:Dr. LI XiaojiangE-mail: xjli@genetics.ac.cn CAS
CAS
 中文
中文




.png)
