Angelman syndrome (AS), characterized by severe mental retardation, developmental delay, ataxia, seizures, speech impairment, autism and happy disposition, is caused by mutation of E3 ubiquitin ligase UBE3A, a critical enzyme involved in proteasome-mediated protein degradation. Increasing evidence demonstrates that overexpression or hyperactivation of UBE3A is associated with autism spectrum disorders (ASDs). ASDs are complex disorders characterized by impairment in social interactions and the occurrence of repetitive behaviors. Thus, both loss and gain of UBE3A functions result in neurodevelopmental and cognitive defects. However, the neuronal functions of UBE3A and the mechanism by which altered expression of UBE3A leads to developmental and cognitive defects are poorly understood.
The research group led by Dr. ZHANG Yongqing at the Institute of Genetics and Developmental Biology, Chinese Academy of Sciences, found that Drosophila mutants of ube3a had excess synaptic boutons and endocytic defects at the neuromuscular junctions. Bone morphogenetic protein (BMP) pathway plays a crucial role in neural development throughout evolution. They showed that Drosophila Ube3a regulates synapse development and function at neuromuscular junctions by ubiquitinating and promoting proteasome-mediated degradation of the type I BMP receptor Tkv, and that the negative regulation of Tkv by Ube3a is conserved in mammalian cells. Negative regulation of BMP signaling by UBE3A suggests a previously unknown molecular mechanism that may underlie the pathogenesis of UBE3A-associated AS and autism.
Their results offer novel insight into the intricate regulation of BMP signaling in the nervous system, and suggest a potential intervention strategy for UBE3A-associated disorders by manipulating BMP signaling via genetic and pharmacological means.
This work was supported by grants from the Ministry of Science and Technology, the Strategic Priority Research Program B of the Chinese Academy of Sciences, the National Science Foundation of China.
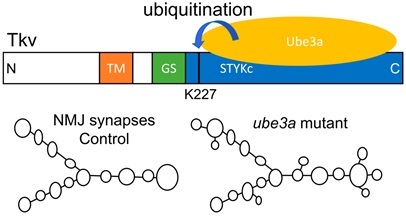
A diagram depicting direct binding and ubiquitination of lysine 227 within the cytoplasmic tail of Tkv by Ube3a and overgrown NMJs caused by increased BMP signaling in ube3a mutants. (Image by IGDB)
Author Contacts:
Dr. YAO Aiyu and ZHANG Yongqing
 A diagram depicting direct binding and ubiquitination of lysine 227 within the cytoplasmic tail of Tkv by Ube3a and overgrown NMJs caused by increased BMP signaling in ube3a mutants. (Image by IGDB)Author Contacts:Dr. YAO Aiyu and ZHANG YongqingE-mail: ayyao@genetics.ac.cn and yqzhang@genetics.ac.cn
A diagram depicting direct binding and ubiquitination of lysine 227 within the cytoplasmic tail of Tkv by Ube3a and overgrown NMJs caused by increased BMP signaling in ube3a mutants. (Image by IGDB)Author Contacts:Dr. YAO Aiyu and ZHANG YongqingE-mail: ayyao@genetics.ac.cn and yqzhang@genetics.ac.cn CAS
CAS
 中文
中文




.png)
