The CRISPR/Cas9 mediated genome editing system has been widely used to introduce targeted mutations in various organisms including plants. When CRISPR/Cas9 DNA integrates into chromosomes, it is often unlinked to the site of modification and can be removed by segregation, leaving a transgene-free mutant plant that carries only the desired DNA sequence changes. However, the commonly used genetic segregation based genome editing method has several disadvantages: the CRISPR/Cas9 DNA transgenic intermediates increases the chance of off-target changes and causes legislation concerns about genetically modified organisms (GMOs); it is still challenging to create mutations in some transformation-recalcitrant species, such as wheat, soybean, sorghum, cotton and woody plants; and it is impossible for vegetatively propagated crops such as potato, banana, and cassava to be modified by this method.
Recently, a research team led by Prof. GAO Caixia in the Institute of Genetics and Developmental Biology of Chinese Academy of Sciences developed two simple and efficient transient CRISPR expression- based genome editing methods.
In this study, the transient expression of CRISPR/Cas9 DNA (abbreviated as TECCDNA) or RNA (abbreviated as TECCRNA) was used for the first time to edit both hexaploid bread wheat (Triticum aestivum L., AABBDD, 2n=6x=42) and tetraploid durum wheat (T. turgidum L. var. durum, AABB, 2n=4x=28). The mutagenesis frequencies of different seven genes in hexaploid bread wheat and tetraploid durum wheat ranged from 1.0%-9.5%. Furthermore, the tissue culture procedures are free of lengthy, costly and labor intensive herbicide selection, and homozygous mutant plants with no detectable transgenes were identified in T0 populations.
The researchers compared the mutagenesis frequencies of TECCDNA, TECCRNA, and conventional DNA integration based genome editing methods in a side-by-side experiment. The mutagenesis frequency of TECCDNA based genome editing (3.3%) was comparable to that of conventional DNA integration based genome editing (3.0%), and the mutagenesis frequency of TECCRNA based genome editing (1.1%) was much lower, which may because RNA was less stable and could be easily degraded compared with DNA. All the mutations obtained by these two genome editing methods transmitted to the next generation faithfully, and the homozygous mutants showed the expected phenotypes.
Nowadays, genome editing has become an important tool not only for gene function study but also for crop genetic improvement. The establishment of two new and efficient genome editing methods relies on the transient expression of CRISPR/Cas9 in wheat callus cells, which is also feasible for nearly all plant species. Moreover, the transient expression of CRISPR/Cas9 RNA can avoid the integration of foreign DNA and improve the biosafety of genome edited products.
“We believe that transient CRISPR expression approach will accelerate molecular breeding studies in wheat, and help to stimulate basic and applied plant genome engineering research”, said Dr. GAO.
This work entitled “Efficient and transgene-free genome editing in wheat through transient expression of CRISPR/Cas9 DNA or RNA” was published in
Nature Communications (
DOI:10.1038/ncomms12617).
This research was supported by the National Natural Science Foundation of China, the Chinese Academy of Sciences, the Ministry of Science and Technology and the Ministry of Agriculture of China.
Figure: Overview of the genome-editing methods based on transient expression of CRISPR/Cas9 DNA or RNA (Image by IGDB).
Contact:
Dr. GAO Caixia
Email: cxgao@genetics.ac.cn
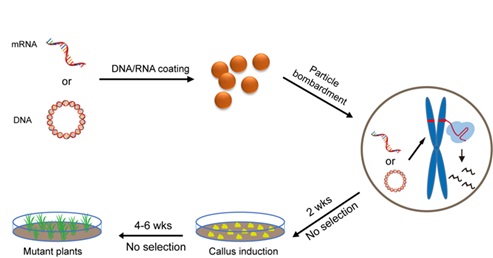 Figure: Overview of the genome-editing methods based on transient expression of CRISPR/Cas9 DNA or RNA (Image by IGDB).Contact:Dr. GAO CaixiaEmail: cxgao@genetics.ac.cn
Figure: Overview of the genome-editing methods based on transient expression of CRISPR/Cas9 DNA or RNA (Image by IGDB).Contact:Dr. GAO CaixiaEmail: cxgao@genetics.ac.cn CAS
CAS
 中文
中文




.png)
