Recently, a team led by Prof. WANG Xiujie at Institute of Genetics and Developmental Biology, Chinese Academy of Sciences (CAS), in collaboration with Prof. YANG Yungui’s team at Beijing Institute of Genomics, CAS, reported a new study demonstrating the function of N6-methyladenosine (m6A) during learning and long-term memory formation.
Long-term memory formation is essential for mammalian behavioral adaptation and intelligence development, especially for human-specific social behaviors. It has been proven that the formation of long-term memory requires de novo mRNA transcription and protein synthesis, which therefore initiate a secondary gene expression wave to facilitate the long-term memory consolidation, yet how such process is regulated remains largely unknown.
N6-methyladenosine (m6A) is the most prevalent mRNA modification in eukaryotic cells, yet its biological importance was just started to be unraveled since 2012. Studies have shown that m6A plays important roles during embryonic development, organogenesis, tumorigenesis, etc., however, its function in regulating learning and memory formation remains elusive.
In this study, the researchers used hippocampus-specific Mettl3 (m6A methyltransferase) conditional knockout mice as a model, systematically studied the function of m6A in adult cognition process.
By applying animal behavior tests and knockout-rescue experiments, the team demonstrated that m6A positively regulates learning efficiency: decreasing or increasing the abundance of m6A modification in hippocampus results in impaired or enhanced learning capacity in mice without affecting their physiological status, brain structure, motor coordination, exploration, thigmotaxis, anxiety level, and short-term memory formation.
Interestingly, such m6A abundance-dependent differences in learning capacity can be compensated by over-dose training, demonstrating that m6A positively regulates the learning efficiency, yet extensive training can overcome m6A-deficiency-induced defects.
Furthermore, the team showed that m6A depletion does not alter either the neuronal intrinsic electrophysiological properties or the synaptic short-term plasticity, but impairs synaptic long-term potentiation. Though m6A is dynamically regulated during the early learning process, the mRNA abundances of m6A-modified genes are unaltered upon Mettl3 knockout. Instead, the team found that m6A enhances the protein translation of genes that are specifically expressed during learning process, hence affecting the long-term memory formation.
The researchers further found that, among wildtype mice, hippocampal METTL3 protein abundance is positively correlated with their learning efficiency: mice that learned fast had significantly higher METTL3 protein abundance in their hippocampus. Such association would be diminished when enough training is given, suggesting that the m6A abundance in hippocampus may be partially responsible for the individual variation in learning efficiency.
This work entitled “METTL3-mediated
N6-methyladenosine mRNA modification enhances long-term memory consolidation” was published in
Cell Research (
DOI: 10.1038/s41422-018-0092-9).
This research was supported by the National Basic Research Program of China, the National Natural Science Foundation of China, the CAS Advance Research Program, and the CAS Strategic Priority Research Program.
Figure. METTL3-mediated m6A modification enhances long-term memory formation. Prolonged training compensates m6A-defeciency-induced learning defects, and overexpression of Mettl3 enhances learning efficacy. (Image by IGDB)
Contact:
Mr. QI Lei
Institute of Genetics and Developmental Biology, Chinese Academy of Sciences
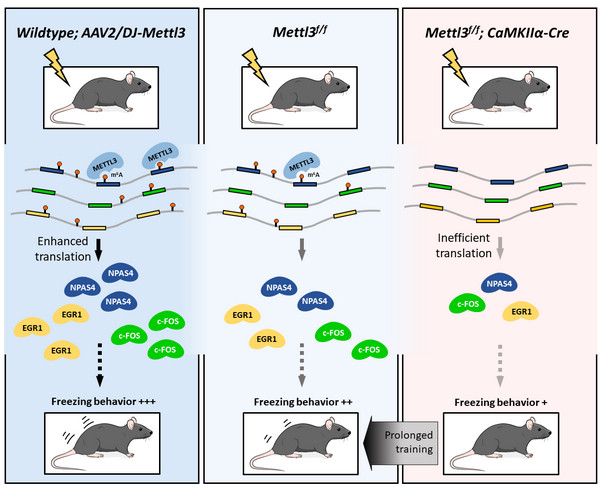 Figure. METTL3-mediated m6A modification enhances long-term memory formation. Prolonged training compensates m6A-defeciency-induced learning defects, and overexpression of Mettl3 enhances learning efficacy. (Image by IGDB)Contact:Mr. QI LeiInstitute of Genetics and Developmental Biology, Chinese Academy of SciencesE-mail: lqi@genetics.ac.cn
Figure. METTL3-mediated m6A modification enhances long-term memory formation. Prolonged training compensates m6A-defeciency-induced learning defects, and overexpression of Mettl3 enhances learning efficacy. (Image by IGDB)Contact:Mr. QI LeiInstitute of Genetics and Developmental Biology, Chinese Academy of SciencesE-mail: lqi@genetics.ac.cn CAS
CAS
 中文
中文




.png)
