Nitric oxide (NO) is an important signaling molecule and modulates multiple biological processes. NO executes its physiological roles mainly through S-nitrosylation, a redox-based protein posttranslational modification by covalently adding an NO group to the thiol group of a cysteine residue. S-nitrosylation was considered as a non-enzymatic process. Emerging evidence indicates that a NO moiety can be transferred from the a transnitrosylase to a target protein, a process named as transnitrosylation. While several transnitrosylases have been characterized in animals and bacteria, yet no transnitrosylase has been found in plants.
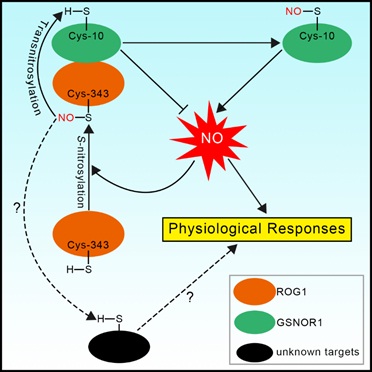
Recently, a team led by Prof. ZUO Jianru at Institutes of Genetics and Developmental Biology (IGDB) of Chinese Academy of Sciences (CAS) and collaborators identified a transnitrosylase, named ROG1, from the model plant species Arabidopsis. They found that ROG1 transnitrosylates GSNOR1, a highly conserved enzyme catalyzing the reduction of the bioactive NO species S-nitrosoglutathione (GSNO). S-nitrosylation of GSNOR1 promotes it autophagic degradation, thereby positively regulating NO signaling. Consistently, the rog1 mutant shows reduced sensitivity to NO under normal growth and oxidative stress conditions.
Unexpectedly, ROG1 is identical to catalase 3 (CAT3). As a non-canonical catalase, ROG1 contains a highly conserved Cys-343 residue essential for its transnitrosylase activity with low catalase activity, while the canonical catalases possess a Thr-343 residue with a higher catalase activity. The substitution of Cys-343 with Thr converts ROG1 into a strong catalase with a significantly lower transnitrosylase activity. Conversely, the substitution of Thr-343 with Cys converts a canonical catalase CAT2 into a strong transnitrosylase with the reduced catalase activity.
A similar mechanism is also found in rice, suggesting that ROG1-like proteins are functionally conserved in plants. ROG1-like proteins are structurally distinguished from transnitrosylases in animals and bacteria, thus representing a unique mechanism in regulating NO signaling in plants.
This study is published in Developmental Cell and was supported by grants from the National Natural Science Foundation of China, CAS and State Key Laboratory of Plant Genomics.
A proposed model of ROG1-regulated NO signaling (Image by IGDB)
Contact:
Prof. ZUO Jianru
Institutes of Genetics and Developmental Biology, Chinese Academy of Sciences
 A proposed model of ROG1-regulated NO signaling (Image by IGDB)Contact:Prof. ZUO JianruInstitutes of Genetics and Developmental Biology, Chinese Academy of SciencesEmail: jrzuo@genetics.ac.cn
A proposed model of ROG1-regulated NO signaling (Image by IGDB)Contact:Prof. ZUO JianruInstitutes of Genetics and Developmental Biology, Chinese Academy of SciencesEmail: jrzuo@genetics.ac.cn CAS
CAS
 中文
中文




.png)
