The phytohormone Abscisic Acid (ABA) is one of the important regulators in plant abiotic stress adaptation. The control of the co-receptor PP2C proteins like ABI1 is the central hub of ABA signaling transduction. Under standard conditions, ABI1 binds to the protein kinase SnRK2s and inhibits their activities. ABA binding to receptor proteins PYR1/PYLs competes with SnRK2s in ABI1 targeting, and thus releasing SnRK2s and activating ABA response.
The team led by Prof. XIE Qi from the Institute of Genetics and Developmental Biology, Chinese Academy of Sciences has long been investigating the ubiquitination, a post-translational modification mechanism, in the regulation of ABA signaling. Their previous work uncovered the ubiquitination mediated endocytosis of PYL4 by the E2-like protein VPS23 and the ABA promotes the degradation of VPS23A by XBAT35, thus release the inhibition of ABA receptor PYL4. However, whether a specific E2 protein, which is required for ubiquitination, is involved in ABA signaling, and how ABA signaling regulates ubiquitination is not well understood.
Recently, they identified a specific E2 enzyme UBC27, which positively regulates plant drought tolerance and ABA response. By IP/MS assay, they identified the ABA co-receptor ABI1 and a RING-type E3 ligase AIRP3 as the interaction proteins of UBC27. They found that UBC27 interacts with and promotes the degradation of ABI1, and activates the E3 activity of AIRP3. AIRP3 works as the E3 ligase for ABI1. Furthermore, ABI1 acts epistasis of UBC27 and AIRP3 and functions of AIRP3 is UBC27-dependent. In addition, ABA treatment induces the expression of UBC27, inhibits the degradation of UBC27, and enhances the interaction between UBC27 and ABI1.
These results uncovered a novel E2-E3 complex in ABI1 degradation, as well as important and complex regulation of ABA signaling by the ubiquitination system.
The paper entitled “The UBC27-AIRP3 ubiquitination complex modulates ABA signaling by promoting the degradation of ABI1 in Arabidopsis” has been published online in
PNAS on October 19, 2020 (
https://doi.org/10.1073/pnas.2007366117).
This research was supported by grants from the National Key R&D Program of China and National Natural Science Foundation of China.
Contact:
Dr. XIE Qi
Institute of Genetics and Developmental Biology, Chinese Academy of Sciences
Figure. A proposed model for UBC27 in ABA signaling pathway (Image by IGDB)
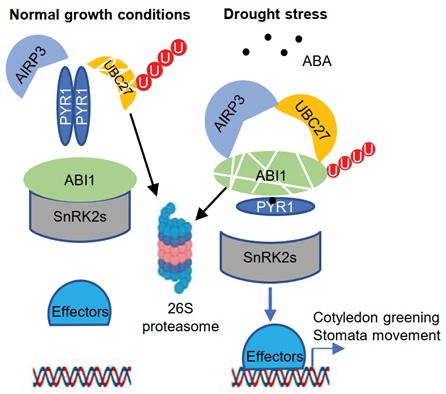 Figure. A proposed model for UBC27 in ABA signaling pathway (Image by IGDB)
Figure. A proposed model for UBC27 in ABA signaling pathway (Image by IGDB) CAS
CAS
 中文
中文




.png)
