Dr. SHUI Guanghou's team from the Institute of Genetics and Developmental Biology of the Chinese Academy of Sciences has recently demonstrated that Ceramide synthases 2 (CerS2) plays a critical role in maintaining hepatic chromosome polyploidization via Mitotic arrest deficient 2 (Mad2) expression during cell division.
In mammalian liver development, existence of polyploidy cells was the general physiological driving force of their physiological functions. Hepatocyte polyploidization is characterized by high proportion of polyploid cells containing more than two sets of homologous chromosomes. Cytokinesis failure is the primary cause of hepatocyte polyploidization. However, there are few studies on ceramide synthase and polyploidy. Ceramide synthase 2 (CerS2), which catalyzes the synthesis of long N-acyl-chain (C22–C24) ceramide, is highly expressed in liver tissue. Hepatic CerS2 dysfunction is related to the pathology of liver diseases, while its mechanism remains unclear.
In this study, the researchers generated hepatocyte-specific CerS2 knockout (cKO) mice, and found that the hepatocytes in cKO mice exhibited with abnormally enlarged nuclei. CerS2 deficiency resulted in marked increases of the hepatic polyploidy population along with substantial steatosis and hepatic carcinoma in 15-month old mice. Lipidomics analysis showed that ceramides and sphingomyelins with different fatty acyls are disordered in hepatocyte nuclei.
They further found that proteins involved in DNA replication, cell cycle and hepatic carcinoma were up-regulated in cKO mice. To further determine which stage of mitosis of cKO hepatocytes was arrested, they used the time-lapse imaging to visualize the cell cycle. Strikingly, CerS2 deficiency increased the proportion of incomplete cytokinesis by up-regulating Mad2 expression and down-regulating Mklp2 and led to M phase arrest.
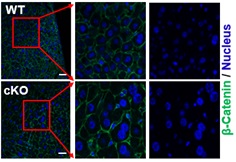
Representative images of immunofluorescence staining for β-catenin (plasma membrane, green) and nuclei (blue) in liver sections of 3-month-old WT and cKO mice (Image by IGDB)
Taken together, these findings highlight that CerS2 or its related sphingolipids play a critical role in the maintenance of liver homeostasis and cell physiology.
This study was published online in Clinical and Translational Medicine on January 29 (Doi:10.1002/ctm2.712).
This work was supported by the National Key Research and Development Program of China and the National Natural Sciences Foundation of China.
Contact:
Dr. SHUI Guanghoud
Institute of Genetics and Developmental Biology, Chinese Academy of Sciences
Email: ghshui@genetics.ac.cn
 Representative images of immunofluorescence staining for β-catenin (plasma membrane, green) and nuclei (blue) in liver sections of 3-month-old WT and cKO mice (Image by IGDB)Taken together, these findings highlight that CerS2 or its related sphingolipids play a critical role in the maintenance of liver homeostasis and cell physiology.This study was published online in Clinical and Translational Medicine on January 29 (Doi:10.1002/ctm2.712).This work was supported by the National Key Research and Development Program of China and the National Natural Sciences Foundation of China.Contact:Dr. SHUI GuanghoudInstitute of Genetics and Developmental Biology, Chinese Academy of SciencesEmail: ghshui@genetics.ac.cn
Representative images of immunofluorescence staining for β-catenin (plasma membrane, green) and nuclei (blue) in liver sections of 3-month-old WT and cKO mice (Image by IGDB)Taken together, these findings highlight that CerS2 or its related sphingolipids play a critical role in the maintenance of liver homeostasis and cell physiology.This study was published online in Clinical and Translational Medicine on January 29 (Doi:10.1002/ctm2.712).This work was supported by the National Key Research and Development Program of China and the National Natural Sciences Foundation of China.Contact:Dr. SHUI GuanghoudInstitute of Genetics and Developmental Biology, Chinese Academy of SciencesEmail: ghshui@genetics.ac.cn CAS
CAS
 中文
中文




.png)
