Three-dimensional bioprinting (3D Bioprinting) combines 3D printer and bioink (usually containing cells and biomaterials) to fabricate tissue/organ-mimicking structures, which is one of the most promising techniques for in vitro human organ generation. However, the commonly used 3D bioprinting systems are usually built on layer-printing-based Cartesian 3D printers, which stacks cells on simple-shaped flat planes with the assistance of biomaterials. Such printing approach is unable to incorporate blood vessel networks during the bioprinting process, therefore facing difficulties in fabricating functional and long-lived complex organs due to the lack of nutrient supply to printed cells. Furthermore, the current bioprinting techniques all rely on the addition of artificial biomaterials in bioinks to fix printed cells, such biomaterials will inhibit the formation of functional cell-cell contact and new blood vessels, thereby interfering the biological functions and long-term survival of the bioprinted products.
To overcome these obstacles, a joint research team led by Prof. WANG Xiu-Jie at the Institute of Genetics and Developmental Biology, Chinese Academy of Sciences, Prof. WANG C.L. Charlie at The University of Manchester and Prof. LIU Yong-Jin at the TsingHua University, reported a novel 3D bioprinting platform.
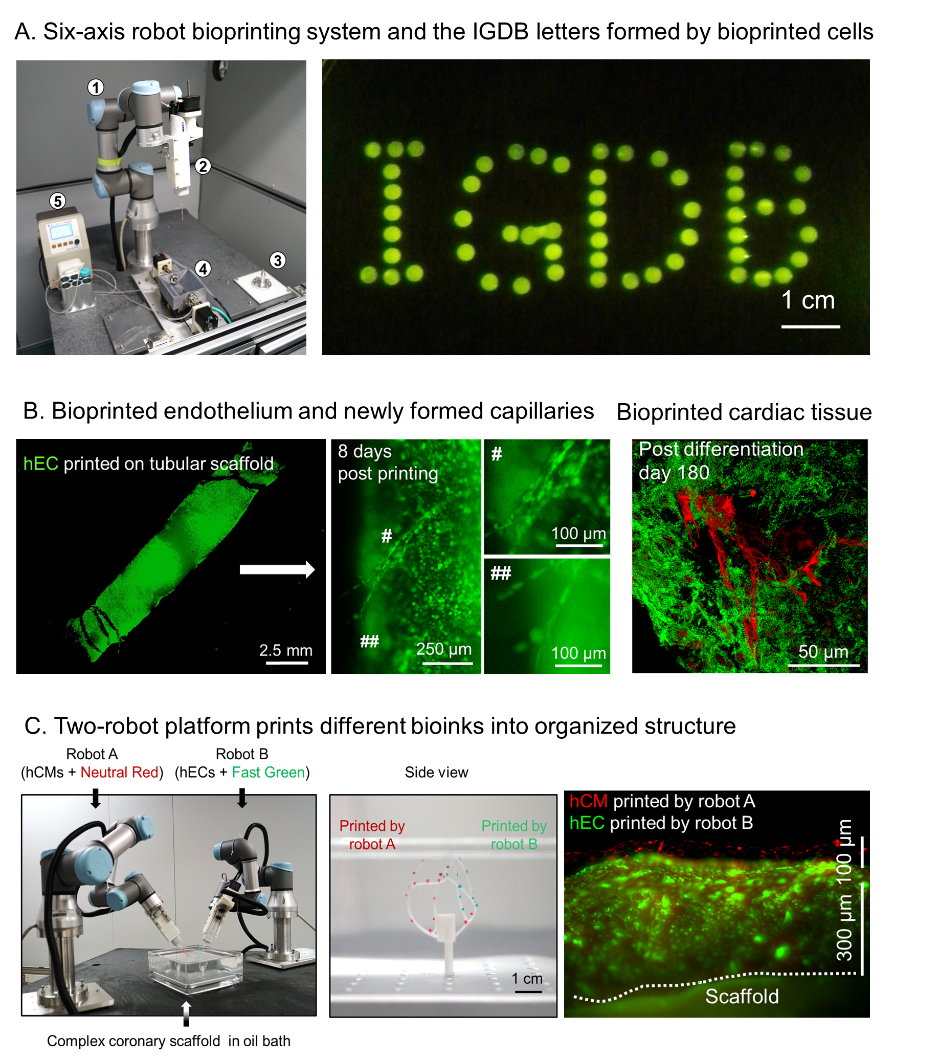 Figure 1. Novel six-axis robot-based bioprinting system and its printing products. (A) Six-axis robot bioprinting platform and the printed IGDB letters composed of eGFP-labeled endothelial cells. (B) Bioprinted artificial blood vessel is capable of forming new capillaries (left and middle, green color is eGFP-labeled endothelial cells). Bioprinted vascularized cardiac tissue (right, green color is cardiac tissue and red color is vascular network). (C) Two-robot cooperation platform (left) can simultaneously print different types of cells onto complex-shaped vascular scaffold (middle) to form patterned cell organization (right). (Image by IGDB)
Figure 1. Novel six-axis robot-based bioprinting system and its printing products. (A) Six-axis robot bioprinting platform and the printed IGDB letters composed of eGFP-labeled endothelial cells. (B) Bioprinted artificial blood vessel is capable of forming new capillaries (left and middle, green color is eGFP-labeled endothelial cells). Bioprinted vascularized cardiac tissue (right, green color is cardiac tissue and red color is vascular network). (C) Two-robot cooperation platform (left) can simultaneously print different types of cells onto complex-shaped vascular scaffold (middle) to form patterned cell organization (right). (Image by IGDB)They creatively converted a six-axis robotic arm into a 3D bioprinter, thereby enabling cell printing from all directions (Figure 1A). To avoid the detrimental effects from biomaterial solidification, researchers also designed an oil-bath-based cell printing system to enable the attachment of printed cells onto blood vessel scaffolds by hydrophobic force, therefore to better keep cell activity and facilitate the formation of cell-cell contacts. Combining the six-axis robot-based bioprinter and oil-bath-based cell printing system, researchers achieved all-directional cell printing on a complex-shaped blood vessel scaffold without causing cell damage or impairing cell proliferation and functions.
Furthermore, inspired by the natural organ developmental process, the team designed a repeated print-and-culture bioprinting strategy: after printing mono- or multi-layers of cells on the blood vessel scaffold, the printed cells would be cultured for certain intervals to induce the formation of cell-cell contact and new blood vessels, then the scaffold and already printed cells were subjected to a new round of bioprinting. In theory, repeating such print-and-culture cycle could generate complex tissue/organs with printed cells interlaced and connected with blood vessel networks to maintain long-term survival and functions.
To demonstrate the feasibility of such strategy, researchers carried out bioprinting experiments using endothelial cell bioink and cardiomyocyte bioink on blood vessel scaffolds, and found that the printed endothelial cells formed intact endothelium as well as new blood vessels and capillaries with the assistance of angiogenic factors (Figure 1B). On the other hand, the printed cardiomyocytes formed gap junctions and resumed rhythmic contraction shortly after printing. By printing the mixture of endothelial cells and cardiomyocytes, researchers generated a piece of vascularized cardiac tissue, which maintained rhythmic beating and alive for at least 6 months (Figure 1B). Taking advantage of the extendibility of the six-axis robots, they further established a two-robot cooperation platform and accomplished simultaneous bioprinting of multiple types of cells on complex-shaped blood vessel scaffolds (Figure 1C).
In summary, compared to conventional methods, the novel 3D bioprinting system reported in this study offers a new strategy to print cells on complex-shaped vascular scaffolds and facilitate angiogenesis among post-printed cells, therefore enables long-term cell survival and demonstrates a feasible way to generate large-scale and functional artificial tissues/organs in vitro.
Paper describing this work entitled “A multi-axis robot-based bioprinting system supporting natural cell function preservation and cardiac tissue fabrication” was published online in Bioactive Materials on February 19, 2022 (doi.org/10.1016/j.bioactmat.2022.02.009), and the research was supported by grants from the CAS Strategic Priority Program and the National Natural Science Foundation of China.
Contact:
Prof. WANG Xiu-Jie
Institute of Genetic and Developmental Biology, Chinese Academy of Sciences

 CAS
CAS
 中文
中文




.png)
