Recently, researchers led by Prof. XU Zhiheng from Institute of Genetics and Developmental Biology, Chinese Academy of Sciences, revealed the evolutionarily conservative and non-conservative regulatory networks during primate interneuron development by single-cell RNA and ATAC sequencing.
Interneurons are highly diverse neurons with the characteristics of different morphologies, connectivity and biological functions. Disturbance of excitatory/inhibitory balance is associated with many neurodevelopmental and neuropsychiatric disorders, such as autism spectrum disorder and schizophrenia. During brain development, only a few interneurons originate and function at local regions, while almost all the rest of interneurons are derived from the ganglionic eminences (GEs), the transitory structures during the embryonic stage. GEs can be divided into three subregions, including lateral, medial and caudal ganglionic eminences (LGE, MGE and CGE). Each subregion expresses specific genes and generates temporally and spatially distinct interneurons targeting to the whole brain. The differences in size and function between primate and rodent brains, and the association of disturbed excitatory/inhibitory balance with many neurodevelopmental disorders highlights the importance to study primate ganglionic eminences development.
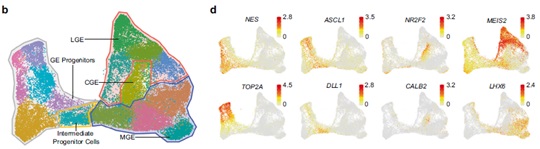
First, using single cell RNA-seq, the researchers confirmed the basic evolutional conservation of cell types between macaque and human, and revealed the cell diversity and developmental trajectory in human and macaque GEs.
Expression profiles of well-recognized marker genes in progenitors, IPCs, MGE, LGE and CGE, visualized by UMAP. (Image by IGDB)
Further analysis on the GE progenitors confirmed the existence of oRGs in human developing GE. Since oRGs are considered
to bethe major driving force for brain volume expansion inprimates, especially human, this would explain the expansion and increased cell density of human GEs, compared with mouse GEs.
Expression of HOPX in human (left) and macaque (right) GE, especially in LGE, as in cortex. Immunofluorescence staining of HOPX at the intersection of GE and cortex. (Image by IGDB)
They also performed scATAC-seq of human GE at GW9 to investigate in more detail the transcription regulatory networks in human developing GEs. They verified the conservation of gene regulatory networks in the developing primate GE and explored the new mechanisms involved in fate determination of different GE regions. For example, human specific gene MIR9-1HG was identified as a potential master regulatory gene in GE progenitors and MGE and a participant in the regulation of differential gene expression between MGE and LGE.
This work described the cell diversity and development lineages of human and Macaca fascicularis by using the scRNA-seq and scATAC-seq. They confirmed the existence of oRGs in primate GEs for the first time and discovered series of genes expressed higher in primate GEs compared with mice. Using scATAC-seq, they established the gene regulatory network for human developing GE and found a human specific gene and its possible regulatory mechanism.
This research provided massive and precious data resources for the study of molecular mechanism in GE development and its related disease.
This work, entitled "Evolutionarily conservative and non-conservative regulatory networks during primate interneuron development revealed by single-cell RNA and ATAC sequencing" (DOI: 10.1038/s41422-022-00635-9), was published in Cell Research March 10, 2022.
Contact:
Dr. XU Zhiheng
Institute of Genetics and Developmental Biology, Chinese Academy of Sciences
 Expression profiles of well-recognized marker genes in progenitors, IPCs, MGE, LGE and CGE, visualized by UMAP. (Image by IGDB)Further analysis on the GE progenitors confirmed the existence of oRGs in human developing GE. Since oRGs are considered to bethe major driving force for brain volume expansion inprimates, especially human, this would explain the expansion and increased cell density of human GEs, compared with mouse GEs.
Expression profiles of well-recognized marker genes in progenitors, IPCs, MGE, LGE and CGE, visualized by UMAP. (Image by IGDB)Further analysis on the GE progenitors confirmed the existence of oRGs in human developing GE. Since oRGs are considered to bethe major driving force for brain volume expansion inprimates, especially human, this would explain the expansion and increased cell density of human GEs, compared with mouse GEs.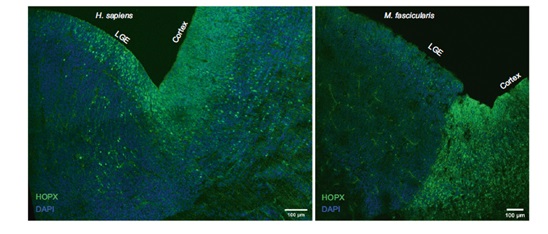 Expression of HOPX in human (left) and macaque (right) GE, especially in LGE, as in cortex. Immunofluorescence staining of HOPX at the intersection of GE and cortex. (Image by IGDB)They also performed scATAC-seq of human GE at GW9 to investigate in more detail the transcription regulatory networks in human developing GEs. They verified the conservation of gene regulatory networks in the developing primate GE and explored the new mechanisms involved in fate determination of different GE regions. For example, human specific gene MIR9-1HG was identified as a potential master regulatory gene in GE progenitors and MGE and a participant in the regulation of differential gene expression between MGE and LGE.This work described the cell diversity and development lineages of human and Macaca fascicularis by using the scRNA-seq and scATAC-seq. They confirmed the existence of oRGs in primate GEs for the first time and discovered series of genes expressed higher in primate GEs compared with mice. Using scATAC-seq, they established the gene regulatory network for human developing GE and found a human specific gene and its possible regulatory mechanism.This research provided massive and precious data resources for the study of molecular mechanism in GE development and its related disease.This work, entitled "Evolutionarily conservative and non-conservative regulatory networks during primate interneuron development revealed by single-cell RNA and ATAC sequencing" (DOI: 10.1038/s41422-022-00635-9), was published in Cell Research March 10, 2022.Contact:Dr. XU ZhihengInstitute of Genetics and Developmental Biology, Chinese Academy of SciencesEmail: zhxu@genetics.ac.cn
Expression of HOPX in human (left) and macaque (right) GE, especially in LGE, as in cortex. Immunofluorescence staining of HOPX at the intersection of GE and cortex. (Image by IGDB)They also performed scATAC-seq of human GE at GW9 to investigate in more detail the transcription regulatory networks in human developing GEs. They verified the conservation of gene regulatory networks in the developing primate GE and explored the new mechanisms involved in fate determination of different GE regions. For example, human specific gene MIR9-1HG was identified as a potential master regulatory gene in GE progenitors and MGE and a participant in the regulation of differential gene expression between MGE and LGE.This work described the cell diversity and development lineages of human and Macaca fascicularis by using the scRNA-seq and scATAC-seq. They confirmed the existence of oRGs in primate GEs for the first time and discovered series of genes expressed higher in primate GEs compared with mice. Using scATAC-seq, they established the gene regulatory network for human developing GE and found a human specific gene and its possible regulatory mechanism.This research provided massive and precious data resources for the study of molecular mechanism in GE development and its related disease.This work, entitled "Evolutionarily conservative and non-conservative regulatory networks during primate interneuron development revealed by single-cell RNA and ATAC sequencing" (DOI: 10.1038/s41422-022-00635-9), was published in Cell Research March 10, 2022.Contact:Dr. XU ZhihengInstitute of Genetics and Developmental Biology, Chinese Academy of SciencesEmail: zhxu@genetics.ac.cn CAS
CAS
 中文
中文




.png)
