Researchers led by Prof. XU Zhiheng from the Institute of Genetics and Developmental Biology of the Chinese Academy of Sciences, in collaboration with Prof. ZHANG Xiaohui’s Lab at Beijing Normal University, revealed the important roles of the scaffold protein plenty of SH3s (POSH) in regulating the assembly of the NMDAR/PSD-95/Shank complex at the postsynaptic density.
POSH regulates assembly of the NMDAR/PSD-95/ Shank complex and synaptic function
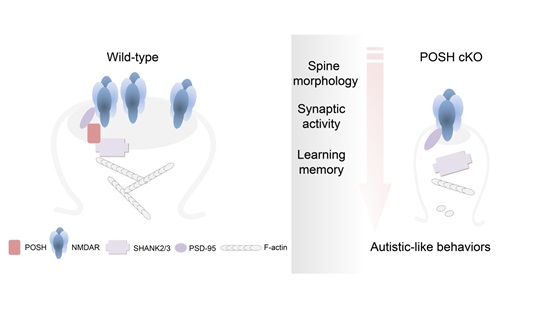
Mutations of Shank family genes are strongly associated with autism spectrum disorders (ASDs) and intellectual disability. N-methyl-D-aspartate glutamate receptor (NMDAR) dysfunction contributes to autistic-like behaviors which may be relieved by pharmacological modulation of NMDAR function. However, the molecular mechanisms involved in the regulation of Shank-mediated NMDAR function is still not clear.
In this study, the researchers found that the scaffold protein POSH clusters at excitatory synapses, together with two other scaffold proteins, PSD95 and SHANK2/3. Mice with brain specific conditional knockout (cKO) of POSH display profound autistic-like behaviors including impairments in social interactions, social communication, repetitive behaviors and deficits in learning and memory.
Combining detailed biochemical and electrophysiological analysis of POSH cKO mouse brain, they found that the normal synaptic clustering of NMDAR/PSD-95/SHANK complex is disrupted at the PSD, accompanied by the abnormal dendritic spine development and aberrant evoked NMDAR-EPSCs and NMDAR-dependent long-term potentiation in hippocampal neurons.
This study not only confirms that POSH is an ASD-associated gene, but also demonstrates that POSH is clustered in PSD with PSD-95 and SHANK2/3, and plays important roles in NMDAR-mediated transmission, spine development, and individual behaviors as well as learning and memory. It further promotes the understanding of the pathogenesis of ASD, and provides a valuable animal model for the research and treatment of ASD in the future.
This work was published in Cell reports on April 5 (DOI:10.1016/j.celrep.2022.110642).
This research was supported by the National Natural Science Foundation of China, and the Ministry of Science and Technology of China, etc.
Contact:
Dr. XU Zhiheng
Institute of Genetics and Developmental Biology, Chinese Academy of Sciences
 POSH regulates assembly of the NMDAR/PSD-95/ Shank complex and synaptic functionMutations of Shank family genes are strongly associated with autism spectrum disorders (ASDs) and intellectual disability. N-methyl-D-aspartate glutamate receptor (NMDAR) dysfunction contributes to autistic-like behaviors which may be relieved by pharmacological modulation of NMDAR function. However, the molecular mechanisms involved in the regulation of Shank-mediated NMDAR function is still not clear.In this study, the researchers found that the scaffold protein POSH clusters at excitatory synapses, together with two other scaffold proteins, PSD95 and SHANK2/3. Mice with brain specific conditional knockout (cKO) of POSH display profound autistic-like behaviors including impairments in social interactions, social communication, repetitive behaviors and deficits in learning and memory.Combining detailed biochemical and electrophysiological analysis of POSH cKO mouse brain, they found that the normal synaptic clustering of NMDAR/PSD-95/SHANK complex is disrupted at the PSD, accompanied by the abnormal dendritic spine development and aberrant evoked NMDAR-EPSCs and NMDAR-dependent long-term potentiation in hippocampal neurons.This study not only confirms that POSH is an ASD-associated gene, but also demonstrates that POSH is clustered in PSD with PSD-95 and SHANK2/3, and plays important roles in NMDAR-mediated transmission, spine development, and individual behaviors as well as learning and memory. It further promotes the understanding of the pathogenesis of ASD, and provides a valuable animal model for the research and treatment of ASD in the future.This work was published in Cell reports on April 5 (DOI:10.1016/j.celrep.2022.110642).This research was supported by the National Natural Science Foundation of China, and the Ministry of Science and Technology of China, etc.Contact:Dr. XU ZhihengInstitute of Genetics and Developmental Biology, Chinese Academy of SciencesEmail: zhxu@genetics.ac.cn
POSH regulates assembly of the NMDAR/PSD-95/ Shank complex and synaptic functionMutations of Shank family genes are strongly associated with autism spectrum disorders (ASDs) and intellectual disability. N-methyl-D-aspartate glutamate receptor (NMDAR) dysfunction contributes to autistic-like behaviors which may be relieved by pharmacological modulation of NMDAR function. However, the molecular mechanisms involved in the regulation of Shank-mediated NMDAR function is still not clear.In this study, the researchers found that the scaffold protein POSH clusters at excitatory synapses, together with two other scaffold proteins, PSD95 and SHANK2/3. Mice with brain specific conditional knockout (cKO) of POSH display profound autistic-like behaviors including impairments in social interactions, social communication, repetitive behaviors and deficits in learning and memory.Combining detailed biochemical and electrophysiological analysis of POSH cKO mouse brain, they found that the normal synaptic clustering of NMDAR/PSD-95/SHANK complex is disrupted at the PSD, accompanied by the abnormal dendritic spine development and aberrant evoked NMDAR-EPSCs and NMDAR-dependent long-term potentiation in hippocampal neurons.This study not only confirms that POSH is an ASD-associated gene, but also demonstrates that POSH is clustered in PSD with PSD-95 and SHANK2/3, and plays important roles in NMDAR-mediated transmission, spine development, and individual behaviors as well as learning and memory. It further promotes the understanding of the pathogenesis of ASD, and provides a valuable animal model for the research and treatment of ASD in the future.This work was published in Cell reports on April 5 (DOI:10.1016/j.celrep.2022.110642).This research was supported by the National Natural Science Foundation of China, and the Ministry of Science and Technology of China, etc.Contact:Dr. XU ZhihengInstitute of Genetics and Developmental Biology, Chinese Academy of SciencesEmail: zhxu@genetics.ac.cn CAS
CAS
 中文
中文




.png)
