Protein sorting in the secretory pathway is essential for cellular compartmentalization and homeostasis in eukaryotic cells. The endoplasmic reticulum (ER) is the biosynthetic and folding factory of secretory cargo proteins. The cargo transport from the ER to the Golgi is highly selective, but the molecular mechanism for the sorting specificity is unclear.
Scientists from the Institute of Genetics and Developmental Biology (IGDB), Chinese Academy of Sciences (CAS) found a new mechanism that guards the ER sorting of LRR-RKs (Leucine-Rich Repeat Receptor Kinases) in Arabidopsis cells.
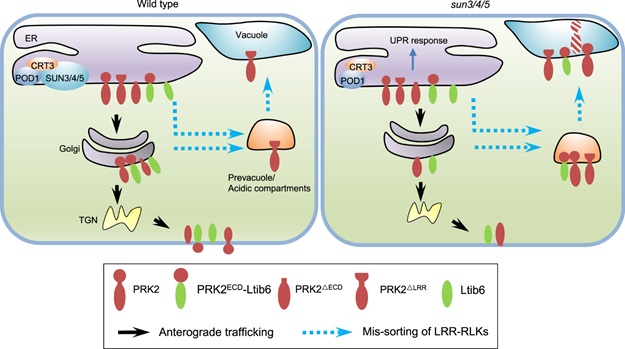
In this study, researchers found three ER localized proteins SUN3, SUN4, SUN5 that regulate ER sorting of LRR-RKs to the plasma membrane. LRR-RKs are extremely important proteins that function in a variety of signaling pathways, including immunity, hormone response. The lost function of SUN3, SUN4, SUN5 lead to BRI1, the brassinosteroid receptor on the plasma membrane, to be localized in acidic compartments. Furthermore, other LRR-RKs, like PRK2, PRK3, MDIS1 which are required for guided pollen tube growth, were also sorted to acidic compartments in sun3/4/5 mutant pollen tubes.
How SUNs regulate the ER sorting of these LRR-RKs? Further studies reveal that the LRR domain of LRR-RKs is the key for their SUN-mediated sorting. The truncation of PRK2 extracellular domain (PRK2ΔECD) allows that PRK2ΔECD escapes from the monitoring of SUNSs and is sorted to the plasma membrane. SUNs recognize the LRR domain to determine whether the LRR-RKs were sorted to Golgi or acid compartments.
Biochemical assays unfold that SUNs interacts with CRT3 and POD1 that are involved in protein maturation and sorting in the ER. These results suggest that POD1-SUNs-CRT3 complex in ER to guarantees the correct fold and sorting of LRR-RKs to the plasma membrane through recognizing their LRR domains.
"This research," said LI Hongju, who led the research, "clarified the ER sorting mechanism of LRR-RKs controlled by the POD1-SUNs-CRT3 complex and deepened the understanding of the complex ER sorting mechanism."
This study was published on Nature Communications recently (DOI:10.1038/s41467-022-30179-w), and supported by the National Natural Science Foundation of China.
SUN3/4/5-POD1-CRT3 on the ER membrane guards the quality control of LRR-RKs (Image by IGDB)
Contact:
Dr. LI Hongju
Institute of Genetics and Developmental Biology (IGDB), Chinese Academy of Sciences
 SUN3/4/5-POD1-CRT3 on the ER membrane guards the quality control of LRR-RKs (Image by IGDB)Contact:Dr. LI HongjuInstitute of Genetics and Developmental Biology (IGDB), Chinese Academy of SciencesEmail: hjli@genetics.ac.cn
SUN3/4/5-POD1-CRT3 on the ER membrane guards the quality control of LRR-RKs (Image by IGDB)Contact:Dr. LI HongjuInstitute of Genetics and Developmental Biology (IGDB), Chinese Academy of SciencesEmail: hjli@genetics.ac.cn CAS
CAS
 中文
中文




.png)
