The plant hormone jasmonate (JA) regulates plant immunity and adaptive growth through orchestrating a genome-wide transcriptional program, which is mainly regulated by the master transcription factor MYC2. It’s well known that MYC2 is repressed by the conserved Groucho/Tup1-like co-repressor TOPLESS (TPL) in the resting state. However, the mechanisms underlying TPL-mediated transcriptional repression and hormone-dependent switching between repression and de-repression still remain unclear.
Previous study reported that TPL interacts with and recruits HISTONE DEACEYLASE 19 (HDA19) to repress C-class gene AG during flower development, through deacetylation of histone proteins. In JA signaling pathway, it is speculated that HDA6 and HDA19 help TPL inhibit the expression of MYC2-targeted JA-responsive genes also by deacetylating histones. However, the fact that both HDA6 and HDA19 loss-of-function mutants showed significantly reduced inducible expression of JA-responsive genes, is contradict with the previously speculated action mechanism of TPL and HDA6/19.
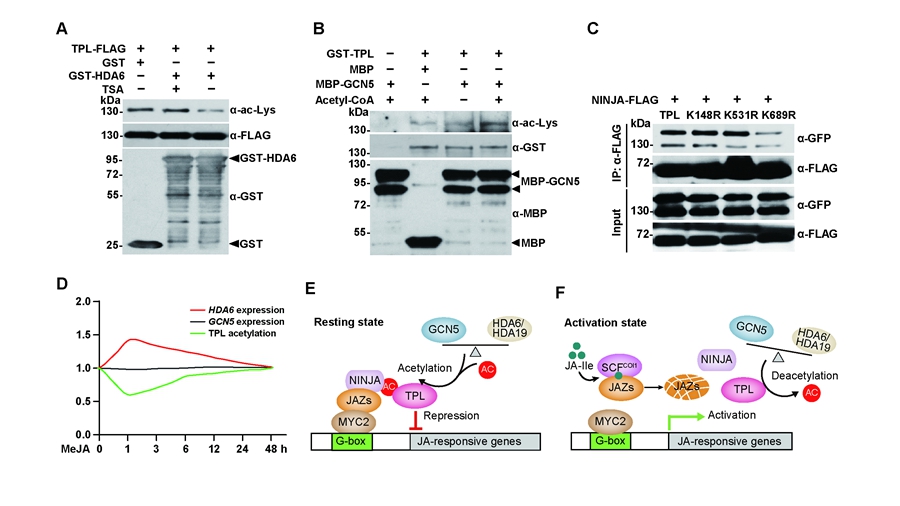
In a study delivered by Prof. Dr. LI Chuanyou’s group at the Institute of Genetics and Developmental Biology (IGDB), Chinese Academy of Sciences (CAS), researchers revealed that HDA6 directly interacts with and deacetylates TPL to positively regulate JA responses, not by presumed targeting histone proteins.
HDA6-mediated TPL deacetylation weakens TPL’s interaction with the adaptor protein NOVEL-INTERACTOR-OF-JAZ (NINJA) and impairs its recruitment to MYC2 target promoters, which finally facilitates gene expression. Conversely, the acetyltransferase GCN5 interacts with and mediates TPL acetylation, which enhances TPL’s interaction with NINJA and promotes its recruitment to MYC2 target promoters to facilitate repression.
In the resting state, the opposing activities of GCN5 and HDA6 maintain TPL acetylation homeostasis, promoting TPL repression activity to repress JA response, which benefits plant growth. In response to JA elicitation, HAD6 expression is transiently induced to decrease TPL acetylation and repressor activity to facilitate JA-responsive gene expression. Thus, the GCN5–TPL–HDA6 module maintains the homeostasis of acetylated TPL, thereby determining the transcriptional state of JA-responsive genes. These findings uncovered a mechanism by which the TPL co-repressor activity in JA signaling being actively tuned in a rapid and reversible manner.
TPL and TPL-related proteins (TPRs) are involved in the repression of a wide range of biological processes, and this study revealed the mechanism by which TPL repression activity is regulated in JA signaling. This study not only correct the presumed action model of TPL/HDA6 in JA signaling pathway, but also add an important perspective to the current understanding of the molecular mechanisms employed by acetyltransferase and deacetylase, whose substrates are not limited to histones.
As a conserved form of protein post-translational modification in animals, plants and microorganisms, non-histone protein acetylation is involved in the regulation of many biological processes. However, compared to the extensive studies of acetylation in regulating non-histone proteins in animals, little is known about the function and regulatory mechanism of non-histone protein acetylation in plants. Therefore, this study provides a good example for studying the function and regulatory mechanisms of non-histone acetylation in plants.
Reversible acetylation and deacetylation of TPL as a switch for JA signaling (Image by IGDB)
This study was published in Molecular Plant (DOI: https://doi.org/10.1016/j.molp.2022.06.014), and was supported by the National Natural Science Foundation of China and the Strategic Priority Research Program of the Chinese Academy of Sciences. Dr. AN Chunpeng, the first author, was funded by the National Postdoctoral Program for Innovative Talents.
Contact:
Prof. Dr. LI Chuanyou
Institute of Genetics and Developmental Biology, Chines Academy of Sciences
Email: cyli@genetics.ac.cn
 Reversible acetylation and deacetylation of TPL as a switch for JA signaling (Image by IGDB)This study was published in Molecular Plant (DOI: https://doi.org/10.1016/j.molp.2022.06.014), and was supported by the National Natural Science Foundation of China and the Strategic Priority Research Program of the Chinese Academy of Sciences. Dr. AN Chunpeng, the first author, was funded by the National Postdoctoral Program for Innovative Talents.Contact:Prof. Dr. LI ChuanyouInstitute of Genetics and Developmental Biology, Chines Academy of SciencesEmail: cyli@genetics.ac.cn
Reversible acetylation and deacetylation of TPL as a switch for JA signaling (Image by IGDB)This study was published in Molecular Plant (DOI: https://doi.org/10.1016/j.molp.2022.06.014), and was supported by the National Natural Science Foundation of China and the Strategic Priority Research Program of the Chinese Academy of Sciences. Dr. AN Chunpeng, the first author, was funded by the National Postdoctoral Program for Innovative Talents.Contact:Prof. Dr. LI ChuanyouInstitute of Genetics and Developmental Biology, Chines Academy of SciencesEmail: cyli@genetics.ac.cn CAS
CAS
 中文
中文




.png)
