Sound information is perceived by the cochlear HCs. Atoh1, a master transcription factor, is both necessary and sufficient for HC development. While no HCs form in
Atoh1 null cochleae, supernumerary HCs emerge upon ectopic
Atoh1 expression. Such an impressing ability of Atoh1 to produce new HCs has made it the best candidate target in regenerating HCs to restore hearing in the damaged cochleae. On August 5, 2022, a study entitled “Three distinct
Atoh1 enhancers cooperate for sound receptor hair cell development” (
https://doi.org/10.1073/pnas.2119850119) was published in
PNAS.
This study was a collaboration between the LIU Zhiyong’s lab at the Institute of Neuroscience, Center for Excellence in Brain Science and Intelligence Technology of the Chinese Academy of Sciences (CEBSIT), and the LU Falong’s lab at the Institute of Genetics and Developmental Biology of the Chinese Academy of Sciences (IGDB).
Atoh1 expression is dynamic and tightly regulated during development, but the underlying mechanisms remain elusive. In the past two decades, the only known Atoh1 enhancer (Eh1, in this study) are believed to be necessary for Atoh1 expression, however, without direct genetic evidence. In the current study, researchers have surprisingly found that deletion of Eh1 failed to impair HC development. The unexpected results suggest that Eh1 is not necessary for HC development and additional novel Atoh1 enhancers exist which can compensate the loss of Eh1 in cochlear HCs. Therefore, researchers sorted pure cochlear HCs, followed by ATAC-seq (assay for transposase-accessible chromatin with high-throughput sequencing) analysis to identify novel Atoh1 enhancers (Figure 1A). Two novel cochlear HC Atoh1 enhancers, Eh2 and Eh3, were identified (Figure 1B). Eh2 and Eh3 are sufficient to drive EGFP reporter expression specifically in cochlear HCs in transgenic models (Figure 1C). Importantly, the three enhancers cooperatively regulate Atoh1 expression and HC development as revealed by single, double, or triple genetic deletion of these enhancers (Figure 1D, E).
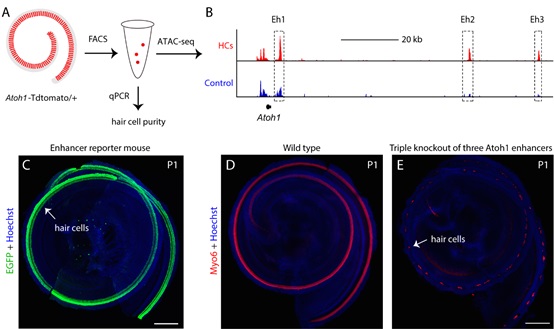
Figure 1. The identification, validation and loss-of-function study of Atoh1 enhancers. (A) Illustration of FACS and ATAC-seq of cochlear HCs. (B) IGV visualization of ATAC-seq peaks in Atoh1 locus. Eh2 and Eh3 are open in HCs, but closed in control cerebellar granule neuron precursors. (C) Eh3 can drive HC-specific EGFP reporter gene expression in cochleae at P1. (D) Hair cells in wild type cochlear. (E) Very few HCs exist in the triple knockout of all three Atoh1 enhancers. (Image by IGDB)
Next, the researchers revealed the genome-wide binding sites of Atoh1 in cochlear HCs. In agreement with the autoregulatory activity, Atoh1 directly binds to Eh1, Eh2 and Eh3 (Figure 2). The findings provide insights into the molecular mechanisms underlying Atoh1 expression regulation in the cochlea HCs and novel potential therapeutic targets for HC regeneration in the damaged cochlea.
Figure 2. Diagram of Atoh1 autoregulation via three Atoh1 enhancer. In wild type cochlear hair cells, Atoh1 directly binds to three enhancers and autoregulates itself. Triple deletion of three Atoh1 enhancers disrupts the Atoh1 autoregulation, leading to the low level of Atoh1 expression and rare formation of hair cells. (Image by IGDB)
This study was supported by Ministry of Science and Technology of China, Chinese Academy of Sciences, National Natural Science Foundation of China, and Science and Technology Commission of Shanghai Municipality.
Contact:
LU Falong
Institute of Genetics and Developmental Biology, Chinese Academy of Sciences, Beijing, China.
LIU Zhiyong
Center for Excellence in Brain Science and Intelligence Technology, Chinese Academy of Sciences, Shanghai, China.
 Figure 1. The identification, validation and loss-of-function study of Atoh1 enhancers. (A) Illustration of FACS and ATAC-seq of cochlear HCs. (B) IGV visualization of ATAC-seq peaks in Atoh1 locus. Eh2 and Eh3 are open in HCs, but closed in control cerebellar granule neuron precursors. (C) Eh3 can drive HC-specific EGFP reporter gene expression in cochleae at P1. (D) Hair cells in wild type cochlear. (E) Very few HCs exist in the triple knockout of all three Atoh1 enhancers. (Image by IGDB)Next, the researchers revealed the genome-wide binding sites of Atoh1 in cochlear HCs. In agreement with the autoregulatory activity, Atoh1 directly binds to Eh1, Eh2 and Eh3 (Figure 2). The findings provide insights into the molecular mechanisms underlying Atoh1 expression regulation in the cochlea HCs and novel potential therapeutic targets for HC regeneration in the damaged cochlea.
Figure 1. The identification, validation and loss-of-function study of Atoh1 enhancers. (A) Illustration of FACS and ATAC-seq of cochlear HCs. (B) IGV visualization of ATAC-seq peaks in Atoh1 locus. Eh2 and Eh3 are open in HCs, but closed in control cerebellar granule neuron precursors. (C) Eh3 can drive HC-specific EGFP reporter gene expression in cochleae at P1. (D) Hair cells in wild type cochlear. (E) Very few HCs exist in the triple knockout of all three Atoh1 enhancers. (Image by IGDB)Next, the researchers revealed the genome-wide binding sites of Atoh1 in cochlear HCs. In agreement with the autoregulatory activity, Atoh1 directly binds to Eh1, Eh2 and Eh3 (Figure 2). The findings provide insights into the molecular mechanisms underlying Atoh1 expression regulation in the cochlea HCs and novel potential therapeutic targets for HC regeneration in the damaged cochlea.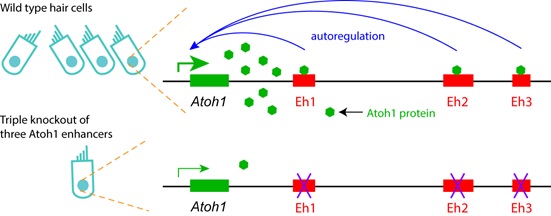 Figure 2. Diagram of Atoh1 autoregulation via three Atoh1 enhancer. In wild type cochlear hair cells, Atoh1 directly binds to three enhancers and autoregulates itself. Triple deletion of three Atoh1 enhancers disrupts the Atoh1 autoregulation, leading to the low level of Atoh1 expression and rare formation of hair cells. (Image by IGDB)This study was supported by Ministry of Science and Technology of China, Chinese Academy of Sciences, National Natural Science Foundation of China, and Science and Technology Commission of Shanghai Municipality.Contact:LU FalongInstitute of Genetics and Developmental Biology, Chinese Academy of Sciences, Beijing, China.E-mail: fllu@genetics.ac.cnLIU ZhiyongCenter for Excellence in Brain Science and Intelligence Technology, Chinese Academy of Sciences, Shanghai, China.E-mail: zhiyongliu@ion.ac.cn
Figure 2. Diagram of Atoh1 autoregulation via three Atoh1 enhancer. In wild type cochlear hair cells, Atoh1 directly binds to three enhancers and autoregulates itself. Triple deletion of three Atoh1 enhancers disrupts the Atoh1 autoregulation, leading to the low level of Atoh1 expression and rare formation of hair cells. (Image by IGDB)This study was supported by Ministry of Science and Technology of China, Chinese Academy of Sciences, National Natural Science Foundation of China, and Science and Technology Commission of Shanghai Municipality.Contact:LU FalongInstitute of Genetics and Developmental Biology, Chinese Academy of Sciences, Beijing, China.E-mail: fllu@genetics.ac.cnLIU ZhiyongCenter for Excellence in Brain Science and Intelligence Technology, Chinese Academy of Sciences, Shanghai, China.E-mail: zhiyongliu@ion.ac.cn CAS
CAS
 中文
中文




.png)
