Embryogenesis is one of the most fundamental and remarkable processes in both animals and plants. It’s amazing that a single maternal egg cell after fertilization can be transformed into an organism with a multi-level body plan only in several weeks. Cell fate transition has largely determined by the related genes’ expression, as well as the epigenetic state, which can affect the genes’ expression. There are conserved and distinct features in cellular process for embryogenesis in animals and plants. Although stacks of studies on animal embryogenesis have been published, gene expression and epigenetic change during plant embryogenesis are still elusive.
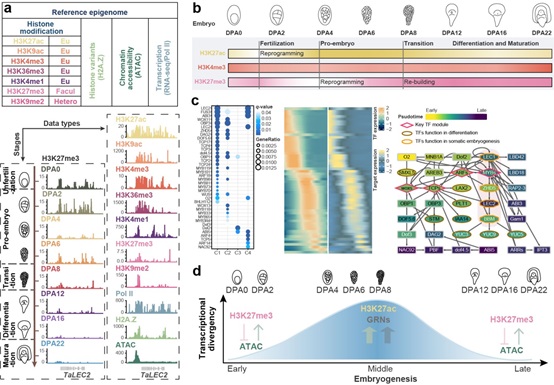
Professor XIAO Jun’s team from the Institute of Genetics and Developmental Biology (IGDB) of the Chinese Academy of Sciences (CAS) focused on the allohexaploid wheat and constructed a “reference epigenome” of wheat embryogenesis. The “reference epigenome” included seven histone modifications (H3K4me1, H3K4me3, H3K9ac, H3K9me2, H3K27ac, H3K27me3, and H3K36me3), occupancy of histone variant H2A.Z and RNA polymerase II, and chromatin accessibility, as well as transcriptomes. The “reference epigenome” profiled the transcription and chromatin state dynamics during embryonic development, and provided clues of regulatory mechanisms underlying cooperation and conflict among sub-genomes within a hexaploid.
According to their recent research, there was a huge transcription and chromatin state change following fertilization in wheat embryo. Histone modification H3K27ac (active gene expression) was decreased at 2 days post anthesis (DPA2) and mainly marked the floral genes. The resetting of H3K27ac may silence these genes and detach the zygotic from the egg cell. H3K27me3 (repress gene expression) was decreased at DPA4, and mainly marked the stem cell niche-related genes. The resetting of H3K27me3 may activate these genes and facilitate cell division. This result suggests that decreasing H3K27ac and H3K27me3 may contribute to zygotic activation (ZGA) in wheat. Interestingly, this epigenetics dynamic pattern was different from that in animals, despite the similar cellular process between animals and plants in the maternal to zygotic transition process (MZT).
At mid-embryogenesis in wheat, the chromatin accessibility and H3K27ac modification were increased and made a permissive chromatin environment. This chromatin state was helpful to transcription factors binding at the cis-regulatory regions of genes. Based on the cis- and trans-regulation features, the researchers built the gene regulatory networks (GRNs) for embryo pattern, which could facilitate the gene function dissection. At late-embryogenesis, the chromatin was condensed again, with H3K27me3 increasing. The condensed chromatin was also found around the differentiated-related genes, which maintained the silence of the genes. This may be why plants can not go through deeper organogenesis like animals.
Token together, the chromatin state was condensed at early- and late-embryogenesis, but accessible at mid-embryogenesis in wheat. This was correlated with the similar-different-similar expression pattern from the comparison among sub-genomes. In addition, different epigenetics modifications and TEs insertion at three sub-genomes may also affect the bias expression of homoeologs. As a huge genome plant, the distal regulation in wheat is more important and complex than the plants with smaller genome sizes such as Arabidopsis and rice.
This work provided an unprecedented epigenomic resource for embryogenesis research in wheat, which can facilitate the functional study of key genes during embryogenesis, especially in ZGA.
This study was published in
Genome Biology entitled "Dynamic chromatin regulatory programs during embryogenesis of hexaploid wheat”(
https://doi.org/10.1186/s13059-022-02844-2). It was supported by the Strategic Priority Research Program of CAS, and the National Natural Science Foundation of China.
Stage-specific transcriptional divergence regulation model in wheat embryogenesis (Image by IGDB)
Contact:
Dr. XIAO Jun
Institute of Genetics and Developmental Biology, Chinese Academy of Sciences
 Stage-specific transcriptional divergence regulation model in wheat embryogenesis (Image by IGDB)Contact:Dr. XIAO JunInstitute of Genetics and Developmental Biology, Chinese Academy of SciencesEmail: jxiao@genetics.ac.cn
Stage-specific transcriptional divergence regulation model in wheat embryogenesis (Image by IGDB)Contact:Dr. XIAO JunInstitute of Genetics and Developmental Biology, Chinese Academy of SciencesEmail: jxiao@genetics.ac.cn CAS
CAS
 中文
中文




.png)
