Acute myocardial infarction (AMI) causes a massive loss of cardiomyocytes, leading to heart failure accompanied by fibrosis, stiffening of the heart and loss of function. Diabetes mellitus (DM) is a well-known risk factor for AMI. Its frequency has steadily increased in AMI patients over the past two decades. Acute hyperglycemia, defined as elevated plasma glucose at hospital admission, was shown as an independent determinant of adverse events in AMI. Acute hyperglycemia leads to a prothrombotic state that exacerbates inflammation and oxidative stress, which adversely affects endothelial maintenance and microcirculatory function. However, apart from the marked increase in glucose at the acute state, little is known regarding the global metabolic dysregulation in this critical time window that could potentially reveal new metabolic targets for therapeutic intervention.
In a recent study delivered by Prof. Dr. SHUI Guanghou’s group at the Institute of Genetics and Developmental Biology (IGDB) of Chinese Academy of Sciences (CAS), Dr. XIA Jing-Gang and Dr. YIN Chun-lin from Xuanwu Hospital reported revealed systemic metabolic dysregulation distinct to acute myocardial infarction associated with diabetes.
They used precise metabolomics to investigate plasma metabolic differences between DM-AMI and non-DM-AMI patients. A diverse array of perturbed metabolic pathways involving carbohydrate metabolism, lipid metabolism, glycolysis, tricarboxylic acid cycle and amino acid metabolism emerged. Precise metabolomics also defined a metabolic signature of afflicted mitochondrial function aggravated by concurrent diabetes in AMI patients. In particular, N-lactoyl-phenylalanine (N-Lac-Phe) and lyso-phosphatidylcholines (LPCs) as key functional metabolites that skewed the metabolic picture of DM-AMI relative to non-DM-AMI.
Precise metabolomics evaluation of distinct plasma metabolome alterations in DM-AMI relative to non-DM-AMI dissects the molecular microenvironment that contributes to compromised cardiac function and worse outcome in DM-AMI. The characteristic metabolic shifts provide new clues and therapeutic targets specific to the treatment of DM-AMI.
This work entited “Precise Metabolomics Defines Systemic Metabolic Dysregulation Distinct to Acute Myocardial Infarction Associated With Diabetes” was published in Arteriosclerosis, Thrombosis, and Vascular Viology on 2 Feb 2023 (DOI:10.1161/ATVBAHA.122.318871).
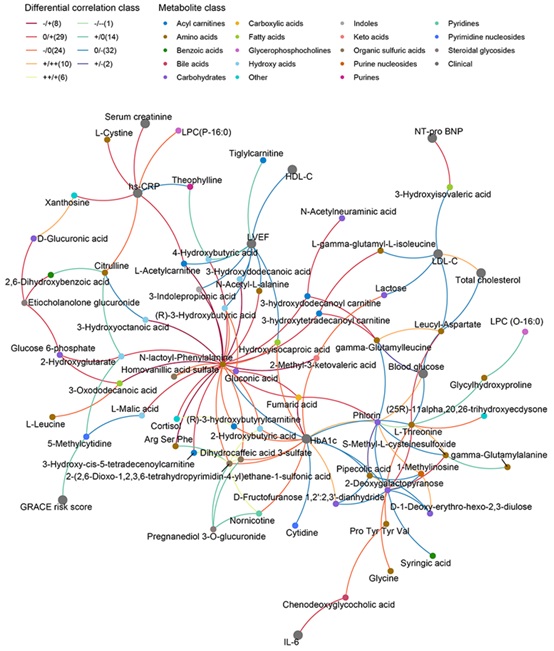
Multiscale embedded gene co-expression network shows the relationships defined by differential correlation analysis (DGCA) among the differential metabolites and clinical indices. (Image by IGDB)
Contact:
Dr. SHUI Guanghou
Institute of Genetics and Developmental Biology, Chinese Academy of Sciences
Email: ghshui@genetics.ac.cn
 Multiscale embedded gene co-expression network shows the relationships defined by differential correlation analysis (DGCA) among the differential metabolites and clinical indices. (Image by IGDB)Contact:Dr. SHUI GuanghouInstitute of Genetics and Developmental Biology, Chinese Academy of SciencesEmail: ghshui@genetics.ac.cn
Multiscale embedded gene co-expression network shows the relationships defined by differential correlation analysis (DGCA) among the differential metabolites and clinical indices. (Image by IGDB)Contact:Dr. SHUI GuanghouInstitute of Genetics and Developmental Biology, Chinese Academy of SciencesEmail: ghshui@genetics.ac.cn CAS
CAS
 中文
中文




.png)
