Interorgan lipid transport is crucial for organism development and the maintenance of physiological function. Drosophila long-chain acyl-CoA synthetase (dAcsl), which catalyzes the conversion of fatty acids into acyl-coenzyme As (acyl-CoAs), plays a critical role in regulating systemic lipid homeostasis. But what happens when this enzyme is deficient, and how does it impact the delicate balance of interorgan lipid transport?
Perturbed lipid homeostasis leads to an array of physiological disorders. Besides being directly related to metabolic diseases such as obesity, diabetes, and cardiovascular diseases, dyslipidemia is also implicated in the pathogenesis of infectious disease. Besides, disruption of lipid homeostasis also leads to developmental defect. Investigating the intricate mechanisms governing systemic lipid homeostasis is, therefore, an important question in both biology and medicine. Interorgan crosstalk is vital in maintaining systemic homeostasis, which relies on the regulation of signal networks and communication across multiple organ.
As lipid metabolic pathways are highly conserved between humans and Drosophila melanogaster, D.melanogaster represents a useful model for the investigation of human metabolic diseases such as obesity and dyslipidemia. The fat body in insects serves as both a metabolic organ and a storage organ, comparable to the liver and/or adipose tissue in vertebrate. Thus, interaction between the fat body and other tissues is very important in the maintenance of overall energy homeostasis in Drosophila. dAcsl is homologous to human ACSL3/ACSL4. Previous functional studies of Drosophila dAcsl have been centered on neurological and developmental aspects, including neurodevelopment, embryonic segmentation, somite development synaptic growth, and neuroblast proliferation. An investigation into the metabolic aspect of dAcsl function has been lacking thus far.
Given the known roles of acyl-CoAs in intermediary lipid metabolism, scientists from the Institute of Genetics and Developmental Biology investigated the effects of dAcsl knockdown on systemic lipid homeostasis and metabolism. Constructing fat body-specific dAcsl knockdowns, the effects on systemic metabolism in Drosophila larva were investigated. The substrate preference of dAcsl was also validated through systematic characterization of acyl-CoA profiles in dAcsl knockdowns. It was discovered that dAcsl knockdown in the fat body led to considerable disruption of overall lipid metabolism and provoked notable endoplasmic reticulum (ER) stress. Besides, abnormalities in carbohydrate metabolism, marked by decreased levels of protein/lipid glycosylation substrates, were also observed. The resultant disruption in protein glycosylation led to abnormal lipoprotein degradation, leading to drastic reductions of circulating lipoproteins in the Drosophila hemolymph that normally convey lipids from the gut to the fat body for storage, and producing an aberrant phenotype of neutral lipid accumulation in the gut. Importantly, this ectopic lipid accumulation was found to significantly reduce the lifespan of flies.
In addition, it was demonstrated that the role of ACSL4 in modulating lipoprotein content is conserved in human HepG2 cells, revealing a previously uncharacterized metabolic aspect of ACSL4 function in maintaining systemic lipid homeostasis and whole-body metabolism. In all, this study uncovers the role of dAcsl, an enzyme governing key intermediary lipid metabolism, in regulating lipoprotein maturation and secretion into the circulation, which in turn alters systemic lipid homeostasis by modulating interorgan lipid transport and mobilization.
This study entitled “Long-chain acyl-CoA synthetase regulates systemic lipid homeostasis via glycosylation-dependent lipoprotein production” is published online in Life Metabolism on 2024.
Figure. Working model for the role of dAcsl inthe regulation of circulating lipoprotein level.
Contact:
Dr. SHUI Guanghou
Institute of Genetics and Developmental Biology, Chinese Academy of Sciences
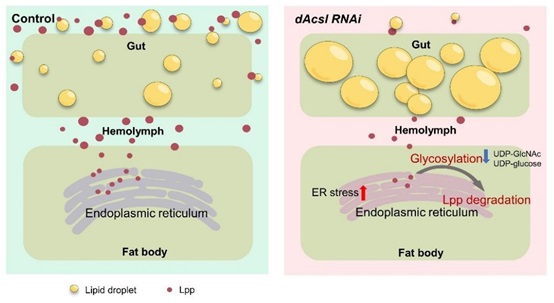 Figure. Working model for the role of dAcsl inthe regulation of circulating lipoprotein level.Contact:Dr. SHUI GuanghouInstitute of Genetics and Developmental Biology, Chinese Academy of SciencesEmail: ghshui@genetics.ac.cn
Figure. Working model for the role of dAcsl inthe regulation of circulating lipoprotein level.Contact:Dr. SHUI GuanghouInstitute of Genetics and Developmental Biology, Chinese Academy of SciencesEmail: ghshui@genetics.ac.cn CAS
CAS
 中文
中文




.png)
