Arginine methylation is an important post-translational modification involved in transcriptional regulation, RNA processing, DNA damage repair, and immune response. Protein arginine methyltransferases (PRMTs) are the evolutionarily conserved protein family responsible for catalyzing protein arginine methylation. Plant PRMTs affect plant development and stress response through transcriptional and post-transcriptional regulation. However, it remains largely unknown whether PRMTs are involved in plant disease resistance.
Chinese scientists established PRMT6-mediated arginine methylation of viral suppressor RNA silencing (VSR) as a novel mechanism of plant immunity against viruses. Their work, entitled "Protein targeting methyltransferase 6 media antiviral immunity in plants", was published in
Cell Host & Microbe on Aug. 5.
The siRNA-mediated antiviral gene silencing plays a key role in plant defense against viruses. VSRs encoded by viruses inhibit antiviral RNA silencing. During plant-virus co-evolution, plants have evolved additional countering strategies to target VSR, such as autophagy and nucleotide-binding-leucine-rich repeat (NLR) containing immune receptor-mediated immunity.
Tomato bush stunt virus (TBSV)-encoded P19 is a well-studied VSR that promotes viral systemic movement by binding to the siRNA duplex. A previous study of Professor CAO Xiaofeng's team found that PRMT6 overexpression could restore the phenotype of overexpressing P19 in Arabidopsis, while PRMT6 knockout exacerbates the phenotype of P19 overexpression. This suggests that PRMT6 in plants may play a role in antiviral responses.
Given that tomatoes are the natural host of TBSV, CAO's team collaborated with Professor LI Feng's team from Huazhong Agricultural University to further investigate the role of PRMT6 in tomato antiviral responses.
Through bioinformatics analysis, the scientists identified a tomato protein, SlyPRMT6, which is homologous to PRMT6 in humans and Arabidopsis. SlyPRMT6 knockout mutants displayed reduced tomato resistance to TBSV infection while SlyPRMT6 overexpression increased the TBSV resistance. This indicated that SlyPRMT6 plays a role in tomato antiviral responses.
Two PRMT6 alleles were then identified in the natural tomato population, showing a significant correlation with PRMT6 expression levels and TBSV resistance. This finding suggested that the allele associated with high expression could be valuable for resistance breeding.
Further analysis demonstrated that PRMT6 interacts with P19, inhibiting its VSR activity by reducing its capacity to bind siRNA. Mechanistic studies revealed that PRMT6 diminishes P19's affinity for siRNA by methylating residues R43 and R115, which is crucial for dimerization and siRNA duplex interaction.
Point mutations and transient expression experiments in Nicotiana benthamiana showed that mutations of R43 and R115 weaken P19's silencing suppressor function. Subsequent yeast two-hybrid and co-immunoprecipitation experiments, along with previous structural analysis of the P19-siRNA complex, revealed that SlyPRMT6 disrupts P19 homodimer formation by methylating arginine 43.
Moreover, methylation of arginine 115 impaired the binding of P19 dimers to siRNA, thereby compromising the silencing suppressor function of P19.
This study unveils a novel mechanism of PRMT6-mediated antiviral immunity, complementing the unknown NLR-mediated immunity and autophagy pathways.
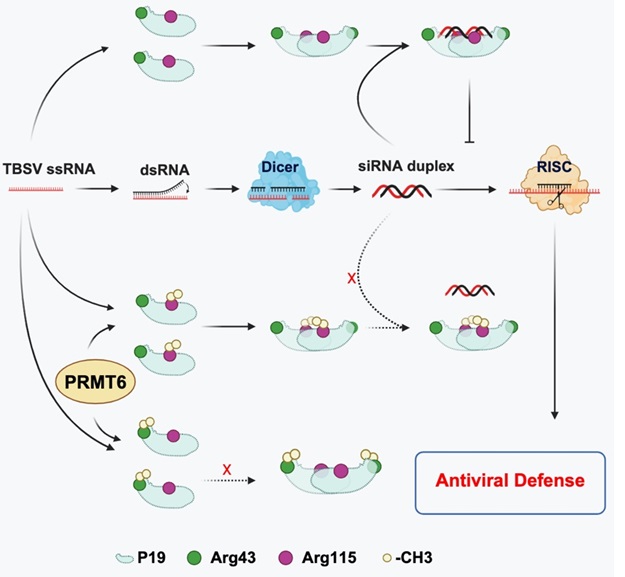
Model of PRMT6-mediated antiviral immunity in plants (Image by IGDB)
Contact:
Dr. CAO Xiaofeng
Institute of Genetics and Developmental Biology, Chinese Academy of Sciences
Email: xfcao@genetics.ac.cn
 Model of PRMT6-mediated antiviral immunity in plants (Image by IGDB)Contact:Dr. CAO XiaofengInstitute of Genetics and Developmental Biology, Chinese Academy of SciencesEmail: xfcao@genetics.ac.cn
Model of PRMT6-mediated antiviral immunity in plants (Image by IGDB)Contact:Dr. CAO XiaofengInstitute of Genetics and Developmental Biology, Chinese Academy of SciencesEmail: xfcao@genetics.ac.cn CAS
CAS
 中文
中文




.png)
