Following spinal cord injury (SCI), fibrotic scars are generated by myofibroblasts, which are typically regarded as an impediment to nerve regeneration. However, the composition, origin and function of fibrotic scars have been a subject of ongoing debate in the field. Previous studies have reported that in non-penetrating spinal cord injuries, myofibroblasts primarily originate from perivascular fibroblasts (PF), whereas in penetrating spinal cord injuries, they mainly arise from meningeal fibroblasts (MF). However, certain studies suggest that in both penetrating and non-penetrating spinal cord injuries, myofibroblasts in fibrotic scars predominantly originate from GLAST+ type A pericytes. Additionally, prior research has demonstrated that the complete removal of fibrous scars often results in a large cavity and is not conducive to injury repair. Conversely, a partial reduction of scars can enhance axon regeneration and functional recovery, suggesting the existence of heterogeneity in the function of fibrous scars following SCI. Understanding the heterogeneous characteristics of fibrotic scars might help to develop strategies for remodeling fibrotic scars after SCI.
In a study published in Nature Communications, a research team led by Profs. DAI Jianwu and ZHAO Yannan from the Institute of Genetics and Developmental Biology of the Chinese Academy of Sciences employed a combination of lineage tracing and single-cell RNA sequencing (scRNA-seq) to demonstrate the heterogeneous distribution, source, and function of meningeal fibroblasts and perivascular fibroblasts in fibrotic scars.
In this study, given that PDFGRβ is expressed in both pericytes and fibroblasts, PDFGRβ-CreER::R26-TdTomato transgenic mice were utilized to enrich PDFGRβ+ cells before and after SCI. scRNA-seq analysis revealed that PDFGRβ+ cells encompassed pericytes/vSMCs and fibroblasts, with the fibroblasts transforming into myofibroblasts after SCI. The team employed Col1a2-CreER::R26-TdT mice to label fibroblasts, NG2-CreER::R26-TdT, and Myh11-CreER::R26 mice to track the cell fate of pericytes/vSMCs. The results further corroborate the contribution of fibroblasts to fibrotic scar formation and rule out the contribution of pericytes/vSMCs.
Subsequently, fibroblasts were classified into Crabp2/Emb+ meningeal fibroblasts (CA-F) and Lama1/Lama2+ pia/perivascular fibroblasts (LA-F) based on the defined markers. Immunostaining indicated that LA-F was located in the parenchymal perivascular space and the meninges inside of CE-F in the uninjured spinal cord. After either transection or crush SCI, CE-F was distributed in the core of the fibrotic scar, surrounded by LA-F. Lineage tracing using Crabp2-CreER::R26-TdT mice confirmed the heterogeneous distribution of LA-F and MF-derived CE-F following SCI. CA-F expressed elevated levels of cholesterol synthesis-related genes and extracellular matrix genes Col1a1 and Fn1, while LA-F highly expressed extracellular matrix genes such as Col4a1 and Lama1. This phenomenon was conserved between mice and monkeys. Additionally, scRNA-seq analysis and in vitro experiments demonstrated that LA-F was implicated in lipid transport and angiogenesis.
The study clarifies the heterogeneity in the cellular composition, origin, distribution, and function of fibrotic scars after spinal cord injury, providing answers to longstanding scientific inquiries in this field and establishing a theoretical foundation for the specific regulation of fibrotic scars.
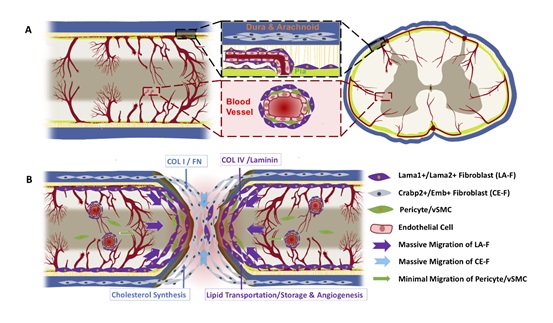
Schematic diagram of fibrotic scar heterogeneity after spinal cord injury (Image by IGDB)
Contact:
Prof. DAI Jianwu and Prof. ZHAO Yannan
Institute of Genetics and Developmental Biology, Chinese Academy of Sciences
 Schematic diagram of fibrotic scar heterogeneity after spinal cord injury (Image by IGDB)Contact:Prof. DAI Jianwu and Prof. ZHAO YannanInstitute of Genetics and Developmental Biology, Chinese Academy of Sciences
Schematic diagram of fibrotic scar heterogeneity after spinal cord injury (Image by IGDB)Contact:Prof. DAI Jianwu and Prof. ZHAO YannanInstitute of Genetics and Developmental Biology, Chinese Academy of Sciences CAS
CAS
 中文
中文




.png)
