The nervous system perceives both environmental stress and internal stimuli and coordinates a range of physiological processes, such as aging and metabolism. When neurons detect mitochondrial damage, they release “mitokine” signals that activate the mitochondrial unfolded protein response (UPRmt) in peripheral tissues, thereby systemically coordinating protein homeostasis and organismal health. Morphogens are a class of secreted signaling factors that form concentration gradients between cells, regulating processes such as cell differentiation, tissue formation, and organ development during embryogenesis. Transforming growth factor-beta (TGF-β), a well-known morphogen, is recognized for its role in regulating growth and development. However, whether TGF-β signaling can function as a mitokine to mediate cell non-autonomous UPRmt activation and participate in the regulation of related physiological functions requires further investigation.
Dr. TIAN Ye's group from the Institute of Genetics and Developmental Biology, Chinese Academy of Sciences, revealed the molecular mechanism of TGF-β signaling pathway coordinating the intestinal UPRmt activation in response to neuronal mitochondrial perturbations, and analyzed the role of the TGF-β signaling pathway in regulating lifespan, immunity, and lipid metabolism in Caenorhabditis elegans.
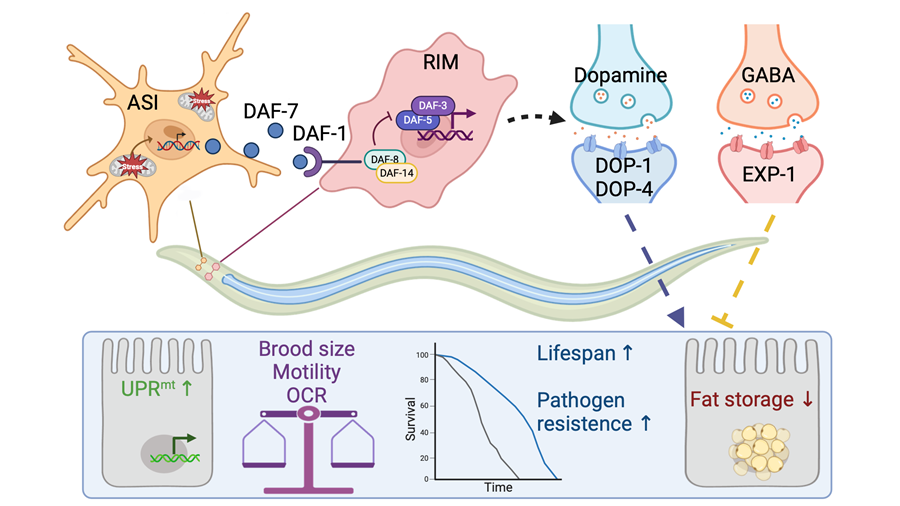
In this study, the researchers identified that the loss-of-function mutation of the TGF-β ligand daf-7 significantly suppressed cell non-autonomous UPRmt induced by neuronal mitochondrial perturbation. Additionally, all components of the TGF-β Dauer signaling pathway are required for the activation of systemic UPRmt. Further investigations revealed that inhibiting daf-7 function in just a pair of ASI neurons was sufficient to block the transmission of mitochondrial stress signals between the nervous system and the intestine. The ligand DAF-7, secreted from ASI neurons, acts on its receptor DAF-1 located on RIM neurons to activate the downstream TGF-β Dauer signaling pathway, thereby inducing UPRmt in intestinal cells. The researchers also found that mitochondrial damage in only one pair of ASI neurons could activate the cell non-autonomous UPRmt. In this model, the activation of UPRmt not only depends on the TGF-β Dauer signaling pathway and the ASI-RIM neuronal axis, but also relies on the neurotransmitter dopamine and is negatively regulated by GABA. The authors further examined the physiological phenotypes of C. elegans with neuronal mitochondrial stress and observed an extended lifespan, enhanced pathogen resistance, and reduced lipid accumulation.
In summary, this study reveals the molecular mechanism by which mitochondrial damage in a pair of ASI neurons can activate the intestinal UPRmt via the TGF-β Dauer signaling pathway. It highlights the critical role of TGF-β in systemically regulating protein homeostasis, adaptive metabolic changes, and aging. Given the high conservation of TGF-β signaling, manipulating TGF-β pathways to coordinate mitochondrial stress responses and promote healthy aging in mammals could be a promising strategy. Additionally, targeting sensory inputs or corresponding receptors in specific neurons offers a potential approach for maintaining mitochondrial homeostasis and balancing lipid accumulation.
This study entitled “
ASI-RIM neuronal axis regulates systemic mitochondrial stress response via TGF-β signaling cascade” was published online in
Nature Communications (
https://doi.org/10.1038/s41467-024-53093-9) on October 19
th, 2024
.Model for ASI-RIM neuronal axis regulates systemic mitochondrial stress response via TGF-β signaling cascade (Image by IGDB)
This work was supported by the National Key R&D program of China, the National Natural Science Foundation of China, the Strategic Priority Research Program of the Chinese Academy of Sciences, the CAS Project for Young Scientists in Basic Research, the China National Postdoctoral Program for Innovative Talents, and the China Postdoctoral Science Foundation.
Contact:
Dr. TIAN Ye
Institute of Genetics and Developmental Biology, the Chinese Academy of Sciences
Email: ytian@genetics.ac.cn
 Model for ASI-RIM neuronal axis regulates systemic mitochondrial stress response via TGF-β signaling cascade (Image by IGDB)This work was supported by the National Key R&D program of China, the National Natural Science Foundation of China, the Strategic Priority Research Program of the Chinese Academy of Sciences, the CAS Project for Young Scientists in Basic Research, the China National Postdoctoral Program for Innovative Talents, and the China Postdoctoral Science Foundation.Contact:Dr. TIAN YeInstitute of Genetics and Developmental Biology, the Chinese Academy of SciencesEmail: ytian@genetics.ac.cn
Model for ASI-RIM neuronal axis regulates systemic mitochondrial stress response via TGF-β signaling cascade (Image by IGDB)This work was supported by the National Key R&D program of China, the National Natural Science Foundation of China, the Strategic Priority Research Program of the Chinese Academy of Sciences, the CAS Project for Young Scientists in Basic Research, the China National Postdoctoral Program for Innovative Talents, and the China Postdoctoral Science Foundation.Contact:Dr. TIAN YeInstitute of Genetics and Developmental Biology, the Chinese Academy of SciencesEmail: ytian@genetics.ac.cn CAS
CAS
 中文
中文




.png)
