The phosphatidylinositol 3-kinase (PI3K)-AKT signaling pathway is one of the most critical and extensively investigated signaling pathways. It is the central regulator of various cellular processes including cell growth, proliferation, metabolism, and survival. Hyperactivation of PI3K-AKT signaling is highly related to a significant number of human diseases, particularly cancers. However, where and how the PI3K-AKT signaling is spatially activated and organized in mammalian cells remains poorly understood.
In a study published in Molecular Cell, a group of researchers led by Dr. HE Kangmin from the Institute of Genetics and Developmental Biology and Dr. FANG Xiaohong from Hangzhou Institute of Medicine, Chinese Academy of Sciences, identified focal adhesions as the subcellular signaling hubs organizing the activation of PI3K-PI(3,4,5)P3-AKT signaling in mammalian cells under basal conditions. This study entitled “Spatial organization of PI3K-PI(3,4,5)P3-AKT signaling by focal adhesions” was published on November 1st, 2024 (https://doi.org/10.1016/j.molcel.2024.10.010).
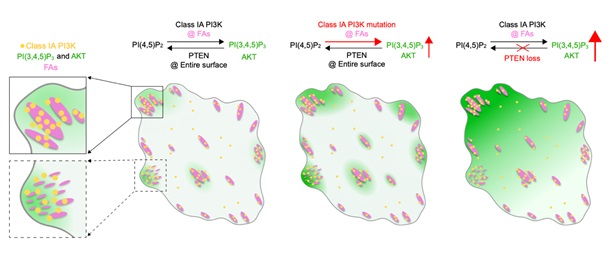
By employing the CRIPSR-Cas9 genome editing tool to fluorescently label endogenous class IA PI3K and imaging the cells using single-molecule quantitative imaging, the researchers discovered that both the catalytic and regulatory subunits of class IA PI3K are preferentially and dynamically recruited to focal adhesions for activation. Activated class I PI3K catalyzes the generation of the critical signaling lipid PI(3,4,5)P3.
To reveal the subcellular distribution and dynamics of trace amounts of PI(3,4,5)P3 in the resting mammalian cells, the they developed a highly sensitive PI(3,4,5)P3 sensor and uncovered that PI(3,4,5)P3 exhibited pronounced local enrichment around focal adhesions. PI(3,4,5)P3 plays critical signaling roles by recruiting and activating various effector proteins, including the serine/threonine protein kinase AKT.
By analyzing the membrane dynamics of endogenous AKT1 using single-molecule quantitative imaging, they revealed that AKT1 molecules are dynamically recruited around focal adhesions for activation in cells under basal conditions.
Furthermore, they found that the spatial recruitment and activation of PI3K-PI(3,4,5)P3-AKT signaling are regulated by focal adhesion kinase. The oncogenic hotspot activation mutation in p110α increases its lipid kinase activity, strongly enhancing the localized generation and thus enrichment of PI(3,4,5)P3 around focal adhesions. In contrast, PTEN loss impedes the depletion of PI(3,4,5)P3 from the cell surface, resulting in a high accumulation of PI(3,4,5)P3 across the entire cell surface while weakening its compartmentalized distribution around focal adhesions.
The canonical view is that class I PI3Ks are primarily activated downstream of agonist-stimulated receptor tyrosine kinases or G-protein-coupled receptors. Therefore, through the utilization of innovative tools and methodologies, this study uncovers a growth-factor independent, compartmentalized organization mechanism for PI3K-PI(3,4,5)P3-AKT signaling in human cancer cells under basal conditions.
This work was funded by the National Key R&D Program of China and the National Natural Science Foundation of China.
A model summarizing the spatial organization of PI3K-PI(3,4,5)P3-AKT signaling by focal adhesions (Image by IGDB).
Contact:
Dr. HE Kangmin
Institute of Genetics and Developmental Biology, Chinese Academy of Sciences
Email: kmhe@genetics.ac.cn
 A model summarizing the spatial organization of PI3K-PI(3,4,5)P3-AKT signaling by focal adhesions (Image by IGDB).Contact:Dr. HE KangminInstitute of Genetics and Developmental Biology, Chinese Academy of SciencesEmail: kmhe@genetics.ac.cn
A model summarizing the spatial organization of PI3K-PI(3,4,5)P3-AKT signaling by focal adhesions (Image by IGDB).Contact:Dr. HE KangminInstitute of Genetics and Developmental Biology, Chinese Academy of SciencesEmail: kmhe@genetics.ac.cn CAS
CAS
 中文
中文




.png)
