Recently, a collaborative research team led by LI Jiayang from the Institute of Genetics and Developmental Biology (IGDB) of the Chinese Academy of Science, Yazhouwan National Laboratory, and China National Rice Research Institute, developed a multiplex gene editing tool named the Cas9-PE system, capable of simultaneously achieving precise editing and site-specific random mutagenesis in rice. Furthermore, by co-editing the ALSS627I gene to confer resistance to the herbicide bispyribac-sodium (BS) as a selection marker, and using Agrobacterium-mediated transient transformation, the researchers successfully achieved transgene-free gene editing in the T0 generation.
This study was published in Trends in Biotechnology (https://doi.org/10.1016/j.tibtech.2024.10.012).
Achieving the aggregation of different mutation types at multiple genomic loci and generating transgene-free plants in the T0 generation is an important goal in crop breeding. Although prime editing (PE), as the latest precise gene editing technology, can achieve any type of base substitution and small insertions or deletions, there are significant differences in efficiency between different editing sites, making it a major challenge to aggregate multiple mutation types within the same plant. Additionally, obtaining transgene-free gene-edited plants using Agrobacterium-mediated transformation also presents considerable difficulties.
In crop breeding, not only precise editing is needed to introduce desired functional mutations, but also a certain degree of random mutagenesis is desirable. This can be used to knock out regulatory genes that cause undesirable phenotypes or affect agronomic traits, and can help generate unexpected superior variations. Based on this need, researchers have developed a Cas9-mediated prime editing (Cas9-PE) system in rice by restoring the single-strand cleavage activity of nCas9 to the double-strand cleavage activity of full-length Cas9. The Cas9-PE system not only retains the ability to perform precise editing with pegRNAs but also gains the new function of targeted random mutation by sgRNAs, thus providing an effective tool for crop breeding.
Previous studies have shown that precise editing of the S627I site in the endogenous acetolactate synthase (ALS) gene can confer herbicide bispyribac-sodium (BS) resistance in rice. In this study, researchers utilized the Cas9-PE system to introduce precise editing of ALSS627I in a multiplex gene editing system to confer BS resistance. A total of 23 rice plants were obtained, among which 7 had edits at the ALSS627I site. Among these, 3 plants were both free of exogenous transgenic components and had precise edits at the ALSS627I site, and 2 of them completed the aggregation of precise editing and random mutations at other target genes. Through this strategy, Cas9-PE successfully produced transgene-free multiplex gene edited plants in the T0 generation.
This work was supported by grants from the National Key R&D Program of China, the CAS Project for Young Scientists in Basic Research, the Agricultural Science and Technology Innovation Program, the National Natural Science Foundation of China.
Figure 1: The Cas9-PE system and strategy for achieving transgene-free plants in the T0 generation in rice (Image by IGDB)
Contact:
Dr. YU Hong
Institute of Genetics and Developmental Biology (IGDB) of the Chinese Academy of Science
Email: hyu@genetics.ac.cn
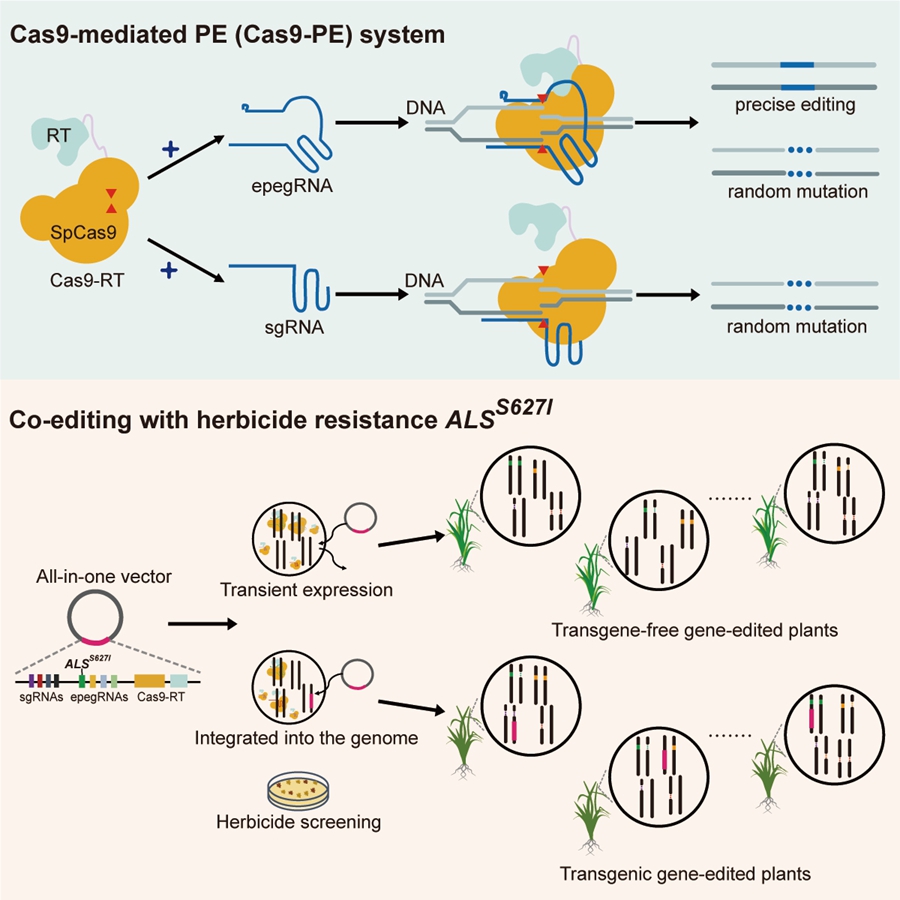 Figure 1: The Cas9-PE system and strategy for achieving transgene-free plants in the T0 generation in rice (Image by IGDB)Contact:Dr. YU HongInstitute of Genetics and Developmental Biology (IGDB) of the Chinese Academy of ScienceEmail: hyu@genetics.ac.cn
Figure 1: The Cas9-PE system and strategy for achieving transgene-free plants in the T0 generation in rice (Image by IGDB)Contact:Dr. YU HongInstitute of Genetics and Developmental Biology (IGDB) of the Chinese Academy of ScienceEmail: hyu@genetics.ac.cn CAS
CAS
 中文
中文




.png)
