On May 7, 2025, WU Zhaofa’s lab from the Institute of Genetics and Developmental Biology, Chinese Academy of Sciences, jointly published an article titled "A high-performance fluorescent sensor spatiotemporally reveals cell-type specific regulation of intracellular adenosine in vivo" in the journal Nature Communications, in collaboration with Jing Wang’s lab from the Peking University School of Pharmaceutical Sciences, and Yulong Li’s lab from the Peking University School of Life Sciences. The study successfully developed HypnoS (Hypersensitive intracellular adenosine Sensor), a genetically-encoded fluorescent sensor for the real-time monitoring of intracellular adenosine (iAdo) in a cell type-specific manner. They monitor iAdo dynamics during seizures or sleep-wake cycles with high spatiotemporal resolution in the brain of living animals.
Extracellular and intracellular adenosine (eAdo and iAdo) form a transmembrane dialogue network through ENT transporters, and their imbalance is closely related to diseases such as neurodegenerative diseases, metabolic syndromes, and tumors. However, due to the limitations of traditional technologies in capturing the millisecond-level dynamic changes of iAdo and its subcellular localization patterns, there are still many controversies regarding the cellular origin of adenosine, its release mechanisms, and the specific processes of transmembrane transport.
Based on circularly permuted enhanced green fluorescent protein (cpEGFP) and adenosine deaminase (PvADA), the research team screened over 3,000 mutants and successfully constructed the novel fluorescent probe HypnoS (named after the "god of sleep" in Greek mythology). This probe exhibits a maximum response amplitude of approximately 900%, features sub-second response kinetics and excellent substrate selectivity, and causes no significant disruption to normal cellular physiological activities. Using HypnoS, the team achieved spatiotemporal visualization of intracellular adenosine at the cellular, tissue, and live animal (fruit fly and mouse) levels.
During pathological processes such as epilepsy, adenosine is considered an important endogenous anticonvulsant factor. By combining the HypnoS probe with in vivo wide-field imaging techniques, the research team visualized the dynamic changes of intracellular adenosine during epileptic seizures in live mouse brains, with the entire cortex as the spatial scale and high-temporal-resolution. This revealed the potential neuroprotective role of intracellular adenosine in epilepsy. Furthermore, the research team specifically expressed HypnoS in neurons and astrocytes and employed in vivo two-photon imaging techniques to parse the dynamic changes of intracellular adenosine in different cell types at single-cell resolution.
In physiological processes such as sleep-wake regulation, adenosine is recognized as a key molecule in sleep homeostasis regulation. The research team, by combining the HypnoS probe with fiber optics and electroencephalogram and electromyogram recordings, captured the phenomenon of increased intracellular adenosine during wakefulness and REM sleep and decreased levels during NREM sleep in the basal forebrain of mice. The study demonstrated that ENT1/2 primarily mediates adenosine release in neurons and promotes adenosine uptake in astrocytes, revealing the division of labor between the two cell types in sleep homeostasis. This finding provides a molecular basis for intervening in sleep disorders through adenosine-level regulation.
The study was supported by institutions, funding, and projects such as the Chinese Academy of Sciences, the National Natural Science Foundation of China, the Ministry of Science and Technology, and the "Youth Talent Support Project" of the Beijing Association for Science and Technology.
Figure. HypnoS: a Hypersensitive intracellular adenosine Sensor (Image by IGDB)
Contact:
Dr. WU Zhaofa
Institute of Genetics and Developmental Biology, Chinese Academy of Sciences
Email: wuzhaofa@genetics.ac.cn
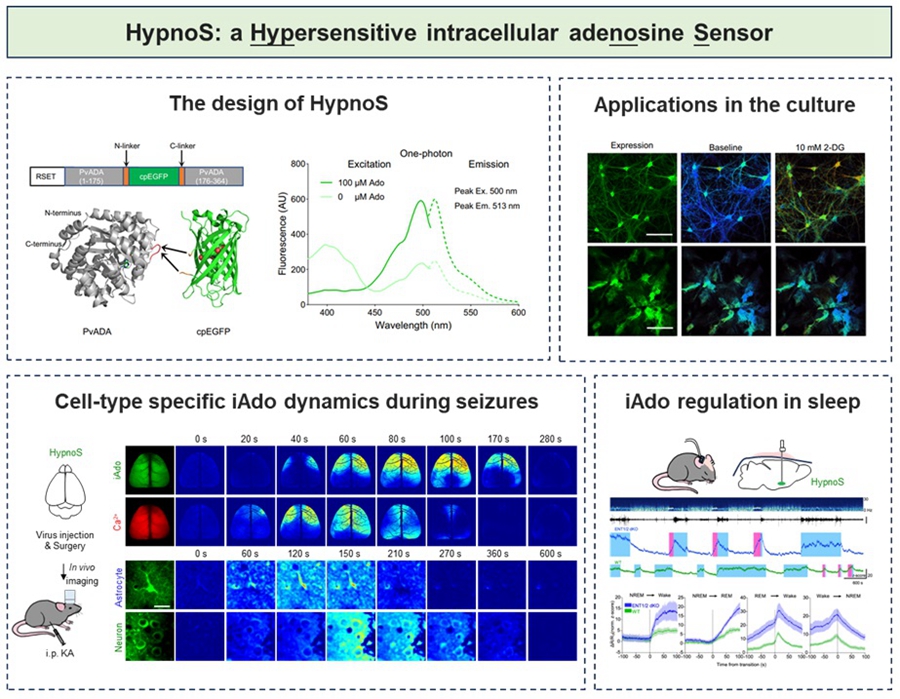 Figure. HypnoS: a Hypersensitive intracellular adenosine Sensor (Image by IGDB)Contact:Dr. WU ZhaofaInstitute of Genetics and Developmental Biology, Chinese Academy of SciencesEmail: wuzhaofa@genetics.ac.cn
Figure. HypnoS: a Hypersensitive intracellular adenosine Sensor (Image by IGDB)Contact:Dr. WU ZhaofaInstitute of Genetics and Developmental Biology, Chinese Academy of SciencesEmail: wuzhaofa@genetics.ac.cn CAS
CAS
 中文
中文




.png)
