Maintaining proteostasis relies on the coordinated action of multiple intracellular mechanisms. However, with the accumulation of aging, genetic mutations, or environmental stress, misfolded proteins may evade immune surveillance and aggregate, thereby triggering neurodegenerative diseases and other protein aggregation disorders. For a long time, the maintenance of proteostasis was regarded as a cell-autonomous process, but recent studies have revealed that inter-tissue signaling plays a crucial role in regulating organism-wide proteostasis.
On August 29, 2025, Dr. TIAN Ye's group from the Institute of Genetics and Developmental Biology of the Chinese Academy of Sciences, in collaboration with Professor Cohen’s team at the Hebrew University, published an online opinion titled “TGF-β signaling as an organismal proteostasis regulator” (DOI: 10.1016/j.tcb.2025.07.008) in Trends in Cell Biology. The article discusses the role and conservation of TGF-β signaling in coordinating proteostasis and explores its potential translational implications.
Cellular and organismal health depend on the integrity of the proteome. The proteostasis network (PN) employs collaborative mechanisms to ensure the correct folding of nascent polypeptides and maintain the stability of mature proteins. Early in life, the PN operates efficiently; however, with advancing age, an overload of misfolded proteins can overwhelm the PN, leading to the formation of toxic protein aggregates that are difficult to clear. This imbalance is closely associated with various late-onset neurodegenerative diseases, including Alzheimer’s disease (AD) and Huntington’s disease (HD).
In response to proteotoxic stress, different organelles within cells have evolved specific stress response pathways, such as the heat shock response (HSR), the endoplasmic reticulum unfolded protein response (UPRER), and the mitochondrial unfolded protein response (UPRmt). These responses primarily function at the cellular level.
A growing body of research indicates that proteostasis is not solely regulated cell-autonomously but can also be influenced across tissues through signaling pathways. Among these, the TGF-β signaling pathway plays a central role.
In the model organism Caenorhabditis elegans, upon sensing mitochondrial stress, the ASI sensory neurons secrete the TGF-β ligand DAF-7. This ligand binds to the DAF-1/DAF-4 receptors on RIM interneurons, activating the DAF-3/DAF-5 transcriptional complex, which in turn initiates the UPRmt in the intestine. This enhances organism-wide stress resistance and extends lifespan.
The TGF-β signaling pathway also plays a critical role in pathogen sensing-induced intestinal UPRER. When neurons detect metabolites from harmful bacteria, they activate intestinal UPRER through a TGF-β-dependent mechanism. This response is significantly attenuated when TGF-β receptors are absent.
Notably, inhibition of TGF-β signaling is not always detrimental, and its activation is not invariably beneficial. Recent studies have shown that inhibiting the nucleolar factor complex (FIB-1/NOL-56) can enhance resistance to toxic protein aggregates via an alternative TGF-β pathway (Figure 2). Specifically, the nucleolar methyltransferase complex (FIB-1/NOL-56) suppresses TGF-β activity, thereby relieving the inhibition on the DAF-3 transcription factor. This subsequently activates HSF-1 and enhances ubiquitin-proteasome system (UPS) function, ultimately alleviating Aβ/polyQ protein toxicity. This mechanism reveals that the nucleolus-TGF-β-HSF-1 axis may represent a novel therapeutic target for proteinopathies.
TGF-β signaling is also involved in proteostasis regulation in mammals, but its effects are context- and tissue-dependent. Overactivation can accelerate muscle protein degradation, leading to muscle atrophy and aging. In cancer cells, TGF-β can induce autophagy-mediated protein degradation, while in fibroblasts, its activity is modulated by autophagy. In human cells, TGF-β participates in extracellular matrix remodeling, mitochondrial fragmentation, and UPRmt activation. These findings suggest that the organism can selectively activate necessary TGF-β signaling pathways to maintain proteostasis according to different environmental and tissue contexts, while suppressing unnecessary responses.
As a central regulatory node in proteostasis, the TGF-β signaling pathway highlights the mechanism of cross-tissue communication in response to proteotoxic stress. This provides new insights for treating protein aggregation-related diseases. Key future questions include: Which specific “messenger” molecules mediate TGF-β signaling from neurons to peripheral tissues? These signaling molecules may include neurotransmitters, neuropeptides, or yet unidentified factors. Once these molecules are identified and their mechanisms elucidated, novel intervention strategies aimed at enhancing proteostasis and delaying the progression of related diseases may be developed.
This work was supported by the National Key Research and Development Program of China, CAS Project for Young Scientists in Basic Research, and the New Cornerstone Science Foundation through the XPLORER PRIZE, etc.
Figure. The regulation of stress response mechanisms and proteostasis signaling in Caenorhabditis elegans by transforming growth factor (TGF)-β. (Image by IGDB)
Contact:
Dr. TIAN Ye
Institute of Genetics and Developmental Biology, the Chinese Academy of Sciences
Email: ytian@genetics.ac.cn
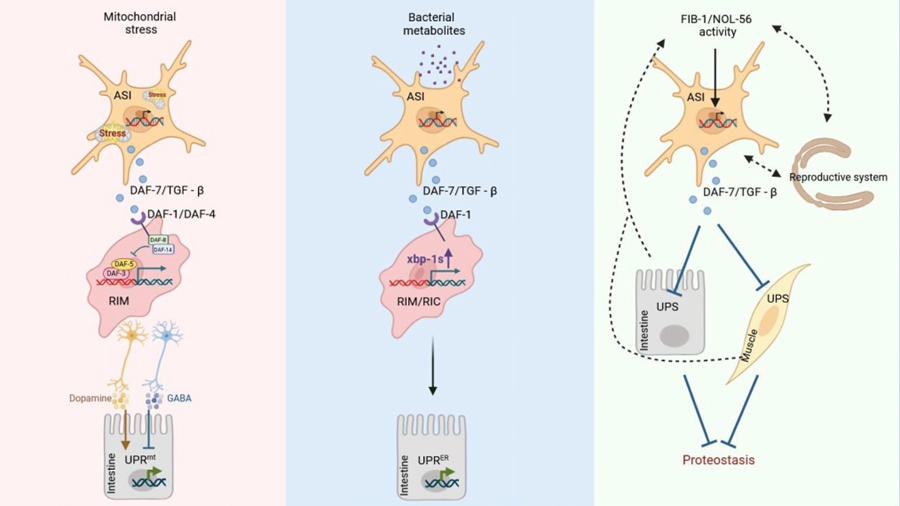 Figure. The regulation of stress response mechanisms and proteostasis signaling in Caenorhabditis elegans by transforming growth factor (TGF)-β. (Image by IGDB)Contact:Dr. TIAN YeInstitute of Genetics and Developmental Biology, the Chinese Academy of SciencesEmail: ytian@genetics.ac.cn
Figure. The regulation of stress response mechanisms and proteostasis signaling in Caenorhabditis elegans by transforming growth factor (TGF)-β. (Image by IGDB)Contact:Dr. TIAN YeInstitute of Genetics and Developmental Biology, the Chinese Academy of SciencesEmail: ytian@genetics.ac.cn CAS
CAS
 中文
中文




.png)
