Microtubules are hollow tubular cytoskeletal structures with a diameter of approximately 25 nm that play central roles in a wide range of fundamental cellular processes. Although microtubule functions are regulated by associated proteins, current knowledge has largely focused on proteins binding the outer surface or ends of microtubules, owing to technical limitations. In contrast, the molecular composition and regulatory roles of proteins residing within the microtubule lumen, an enclosed and spatially constrained compartment, remain poorly understood. While recent advances in cryo-electron microscopy have enabled significant progress in the study of lumen proteins in ciliary doublet microtubules, systematic investigations of cytoplasmic singlet microtubule lumen proteins, which are most widely present in cells, have been notably lacking.
On December 31, 2025, the research group led by Prof. MENG Wenxiang at the Institute of Genetics and Developmental Biology, Chinese Academy of Sciences, developed a capture strategy for singlet microtubule lumen proteins by integrating proximity-dependent biotin identification (Bio-ID) with mass spectrometry, enabling systematic screening and identification of proteins residing within the microtubule lumen.
Using this platform, the study further investigated the regulatory mechanisms of the singlet microtubule luminal microenvironment, focusing on JPT2 as a representative factor previously unrecognized as a microtubule lumen protein. The authors demonstrate that JPT2 directly binds microtubules and enters the microtubule lumen in an intrinsic structure–dependent manner, suggesting that luminal entry may occur through the open ends of microtubules. Within this confined compartment, JPT2 modulates the accessibility of the α-tubulin acetyltransferase MEC17, thereby contributing to the regulation of luminal homeostasis and fine-tuning microtubule acetylation levels.
Further analyses reveal that the luminal localization of JPT2 is highly sensitive to the structural state of microtubules and is markedly reduced upon paclitaxel treatment, indicating that drug-induced conformational changes or steric constraints can restrict protein entry into the lumen. Beyond pharmacological perturbations, these findings further suggest that the distribution of luminal proteins may be shaped by steric competition or selective entry mechanisms among intraluminal components, pointing to a more intricate and dynamic regulatory landscape within the microtubule interior.
Together, this study identifies JPT2 as a key organizer of the singlet microtubule lumen and establishes a conceptual and technical framework for spatial and functional analyses of microtubule inner proteins, highlighting the microtubule lumen as an active regulatory environment rather than a passive structural void.
This work was published online in PNAS (https://doi.org/10.1073/pnas.2520123123) entitled “Systematic Identification of Microtubule Lumen Proteins Reveals a Taxane-Sensitive Luminal Resident JPT2 Regulating MEC17 Accessibility.”
This work was supported by the National Key Research and Development Program of China and the National Natural Science Foundation of China.
Figure. Establishment of the Singlet MIPs Screening System (Imaged by IGDB)
Contact:
Prof. MENG Wenxiang
Institute of Genetics and Developmental Biology, Chinese Academy of Sciences
Email: wxmeng@genetics.ac.cn
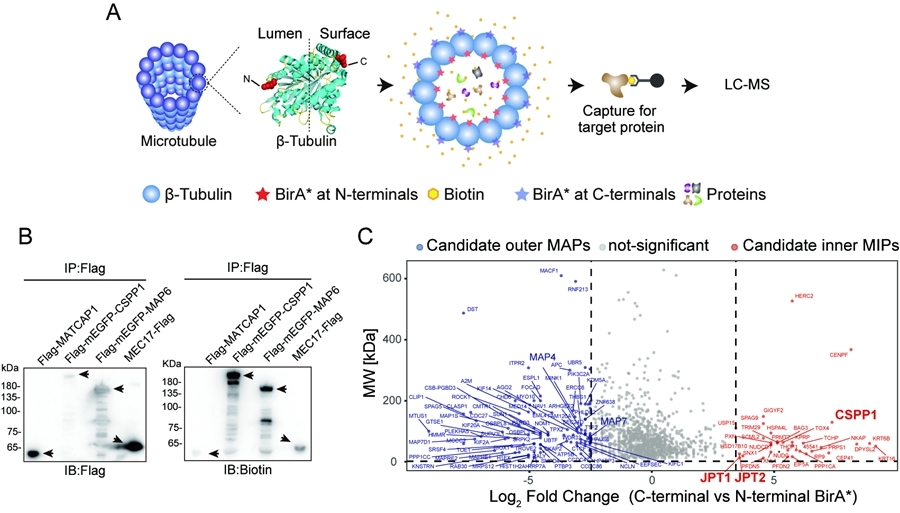 Figure. Establishment of the Singlet MIPs Screening System (Imaged by IGDB)Contact:Prof. MENG WenxiangInstitute of Genetics and Developmental Biology, Chinese Academy of SciencesEmail: wxmeng@genetics.ac.cn
Figure. Establishment of the Singlet MIPs Screening System (Imaged by IGDB)Contact:Prof. MENG WenxiangInstitute of Genetics and Developmental Biology, Chinese Academy of SciencesEmail: wxmeng@genetics.ac.cn CAS
CAS
 中文
中文




.png)
