Intracellular transport is a highly regulated multi-step process which involves recognition and loading of cargo, directed movement of the motor-cargo complex along cytoskeletal tracks, unloading and release of the cargo at its final destination. Dynein/dynactin, the minus end-directed microtubule motor, recognizes its cargoes through interactions between its subunits and adaptor proteins. The molecular events regulating dissociation of the retrograde motor from its vesicular cargoes at their final destination remain to be elucidated.
In collaboration with research groups led by Prof. Changlin Tian (University of Science and Technology of China) and Dr. Weimin Gong (Institute of Biophysics, Chinese Academy of Sciences), Dr. Jia-Jia Liu and her colleagues at the Institute of Genetics and Developmental Biology, Chinese Academy of Sciences found that PtdIns4P, a phospholipid enriched in Golgi membrane, plays an important role in SNX6 retromer-mediated vesicular transport from endosomes to the trans-Golgi network (TGN).
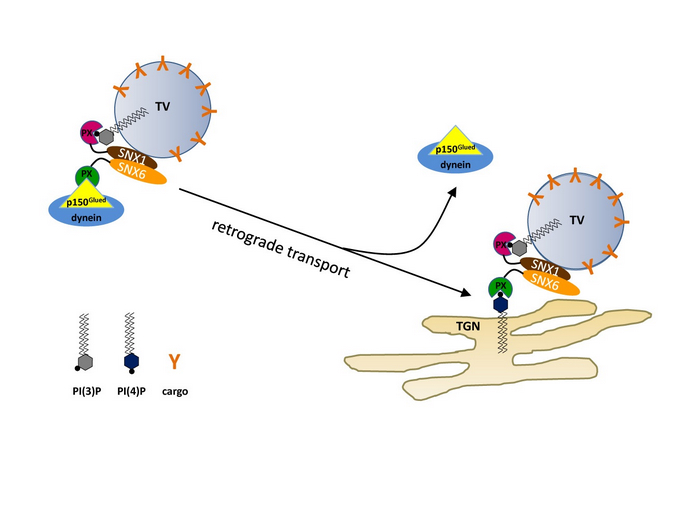
As cargo adaptor for dynein/dynactin, SNX6 binds to p150Glued through its PX domain and to SNX1 through its BAR domain, linking the retromer complex and the retrograde motor at the cytoplasmic side of vesicular cargoes. The PX domain of SNX6 preferentially binds to PtdIns4P in vitro. Acute depletion of PtdIns4P from Golgi membrane causes peri-Golgi accumulation of the retromer cargo CI-MPR, and addition of PtdIns4P restores its Golgi distribution. PtdIns4P specifically inhibits the protein-protein interaction between SNX6 and p150Glud, and incubation of PtdIns4P-containing liposomes with transport vesicles isolated from cells leads to dissociation of the motor complex from the retromer.
This research is the first ever to demonstrate that an organelle-specific phospholipid can regulate precise unloading of vesicular cargoes at the target membrane through its regulation of motor-cargo interaction. In addition, this research also shows that PtdIns4P plays a critical role in retrograde trafficking of tranferrin and its receptor, another dynein cargo, from endosomes to the endocytic recycling compartment (ERC), by regulating the interaction between SNX4 and the dynein light chain-interacting protein KIBRA, suggesting that phospholipid-regulated motor-cargo interaction provides a general mechanism for retrograde vesicular transport.
The related paper was published online on March 24, 2013 in Nature Cell Biology (DOI: 10.1038/ncb2710). This research was supported by grants from the National Natural Science Foundation of China, Ministry of Science and Technology and Chinese Academy of Sciences.
Scientists discover a novel mechanism for cargo release by the retrograde motor dynein/dynactin
(Image by NIU Yang etc.)
Figure. A working model for PI(4)P-regulated release of retromer-associated vesicular cargo by dynein/dynactin at the TGN. The Vps subcomplex of the retromer is omitted for clear illustration of the mechanistic role of the SNX subcomplex in motor-cargo interaction. When retromer-associated vesicular cargoes reach the TGN membrane, PI(4)P weakens the interaction between SNX6 and dynactin p150Glued either by inducing a protein conformational change in SNX6 to modulate its affinity for p150Glued, or by directly competing against p150Glued for the same binding site in the PX domain, thus facilitating the dissociation of the motor from retromer-associated vesicles. TV: transport vesicle.
AUTHOR CONTACT:
LIU Jia-Jia, Ph.D.
Institute of Genetics and Developmetnal Biology, Chinese Academy of Sciences, Beijing, China.
E-mail: jjliu@genetics.ac.cn



