Cellulose is an important natural resource of plant cell wall with great economic value. The crystallographic structure is highly associated with its physicochemical property and uses. However, the mechanism about how cellulose is assembled is poorly understood. Except the self-assembly model, it is unknown that whether this process requires the involvement of other components. COBRA is an important protein for its involvement of cellulose biosynthesis, which has been reported in 2001. But the underlying molecular basis for this protein remains elusive.
Brittle culm1 (BC1) is a COBRA-like protein. Map-based cloning of the corresponding gene has been reported by Prof. Jiayang Li’s group in 2003. It was characterized as a player for secondary wall cellulose formation. Recently, scientists in Dr. Yihua Zhou’s group and Prof. Jiayang Li’s group from Institute of Genetics and Developmental Biology, Chinese Academy of Sciences, and in Prof. Qian Qian’s group from China National Rice Research Institute, Chinese Academy of Agricultural Sciences reported BC1 functioning in cellulose assembly through characterization of its biochemical activities, subcellular behavior, and genetic relationship with CESAs via several combined approaches.
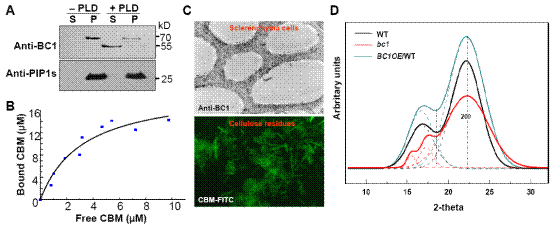
BC1 is a glycosylphosphatidylinositol (GPI) anchored protein and can be released into cell walls, especially the secondary cell wall by removal of the GPI anchor. A carbohydrate-binding module (CBM) is present at its N-terminus, which was demonstrated to bind specifically to crystalline cellulose. This binding activity and cell wall-localization are critical for BC1 function based on in vivo and in vitro analyses. It was further found that the CBM domain and the GPI modification contribute together to the cell wall localization of BC1.
Although BC1 is highly coexpressed with secondary wall CESAs, the genetic evidence suggest BC1 downstream of CESAs’ action. Wide-angle X-ray scattering (WAXS) analysis revealed that bc1 mutant has a decrease in crystallite width of cellulose. Varied crystallinity was found in the CBM-mutated BC1s and overexpression lines, consistent with the in vitro binding assay.
This work with Lifeng Liu and Keke Shangguan as the co-first author has been online published on PLoS Genetics (DOI:10.1371/journal.pgen.1003704). This research was supported by grants from Ministry of Science and Technology, National Natural Science Foundation of China, and Ministry of Agriculture of China.
AUTHOR CONTACT:
Yihua Zhou, Ph.D.
Institute of Genetics and Developmental Biology, Chinese Academy of Sciences, Beijing, China.
E-mail: yhzhou@genetics.ac.cn
(Image by Liu Lifeng etc.)
Figure. BC1 interacts with cellulose and affects microfibril crystallinity. (A) Protein blotting of buffer (-PLD)- or PLD (+PLD)-treated membrane proteins with anti-BC1, demonstrating that BC1 is a GPI anchored protein. S, supernatant and P, pellet fractions. (B) Dissociation constant value for CBM binding to rice crystalline cellulose obtained from a representative experiment. (C) Immunogold labeling of BC1 in cells with thickening cell walls (up panel) and Immunochemical staining of rice crystalline cellulose with recombinant CBM (low panel). (D) The representative two-dimensional scattering images of the indicated rice plants generated by WAXS.



