Huntington’s disease (HD) is an inherited neurodegenerative disease caused by a polyglutamine (polyQ) expansion of >37 glutamines in the HD protein huntingtin (htt). In HD, degeneration occurs preferentially in striatal neurons and extends to other brain regions as the disease progresses. One possible explanation for this is that mutant proteins preferentially affect the specific structures and biological properties of neurons.
Considering that neuronal cells have unique, long neuronal processes and synapses that may be vulnerable to toxic proteins and insults, many studies have focused on synaptic function, revealing that synaptic dysfunction is the common pathological event in a variety of neurodegenerative diseases; however, because of the widespread subcellular distribution of mutant htt and the wide range of its toxic effects, the contribution of mutant htt in synapses to the development of neurological symptoms in HD remains unknown.
Many genetic mouse models of Huntington's disease (HD) have established that mutant huntingtin (htt) accumulates in various subcellular regions to affect a variety of cellular functions, but whether and how synaptic mutant htt directly mediates HD neuropathology remains to be determined. Recently, researchers in Dr. Xiaojiang Li’s group from both Emory University and Institute of Genetics and Developmental Biology, Chinese Academy of Sciences found a typical role of synaptic mutant htt to mediate HD neuropathology.
They generated transgenic mice that selectively express mutant htt in the presynaptic terminals. Although it was not overexpressed, synaptic mutant htt caused age-dependent neurological symptoms and early death in mice as well as defects in synaptic neurotransmitter release. Mass spectrometry analysis of synaptic fractions and immunoprecipitation of synapsin-1 from HD CAG150 knockin mouse brains revealed that mutant htt binds to synapsin-1, a protein whose phosphorylation is critical for neurotransmitter release. They found that polyglutamine-expanded exon1 htt binds to the C-terminal region of synapsin-1 to reduce synapsin-1 phosphorylation. Those findings point to a critical role for synaptic htt in the neurological symptoms of HD, providing a new therapeutic target.
This work was mainly performed by the first author Qiaoqiao Xu at Emory University while she was a visiting graduate student from Tongji Medical College, Huazhong University of Science and Technology. She continued some work at Institute of Genetics and Developmental Biology, Chinese Academy of Sciences as a visiting student. A PhD. student Xudong Liu at Institute of Genetics and Developmental Biology, Chinese Academy of Sciences also made contributions to the work. This work has been online published on J. Cell Biology (doi: 10.1083/jcb.201303146).
AUTHOR CONTACT:
Xiaojiang Li, Ph.D.
Emory University
Institute of Genetics and Developmental Biology, Chinese Academy of Sciences, Beijing, China.
Correspondence to Shihua Li: shihual@genetics.emory.edu; or Xiao-Jiang Li:
xli2@emory.edu
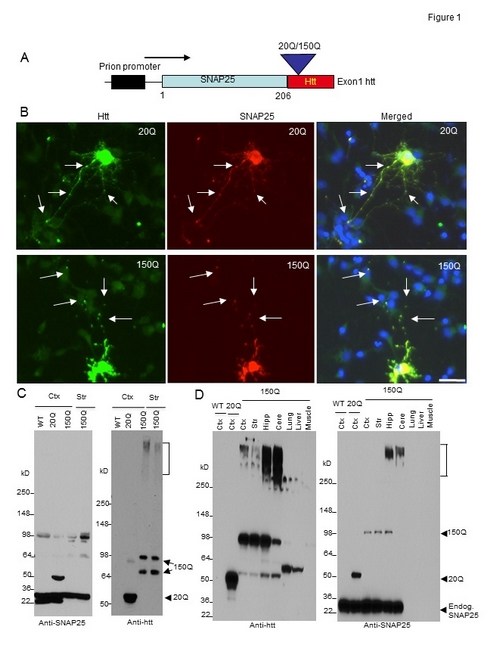
Figure. Generation of transgenic mice expressing N-terminal htt. (A) DNA structure of the transgene for expression of exon1 htt (1–67 amino acids) containing an additional 20Q or 150Q, which is fused to the C terminus of SNAP25 (SNAP25-20Q or SNAP25-150Q) and expressed under the control of the mouse prion promoter. (B) Expression of transfected SNAP25-20Q or SNAP25-150Q in cultured neurons from the rat striatum. The cells were labeled by antibodies to htt (green, mEM48) and SNAP25 (red) and Hoechst to the nuclei (blue). Arrows indicate synapselike puncta. Bar, 10 µm. (C) Western blot analysis of tissue lysates from the cortex (Ctx) and striatum (Str) of wild-type (WT), SNAP25-20Q (20Q), and SNAP25-150Q (150Q; line-8) mice at the age of 4 mo. The blots were probed with antibodies to SNAP25 (left) or htt (right). SNAP25-150Q and its degraded product (double arrows), aggregated htt (bracket), and SNAP25-20Q (arrowhead) are indicated. Anti-SNAP25 also labeled a nonspecific band at 98 kD in WT, as it was not labeled by anti-htt. (D) Western blotting analysis of brain regions (cortex; striatum; Hipp, hippocampus; Cere, cerebellum) and peripheral tissues (lung, liver, and muscle) shows a restricted expression of transgenic mutant htt in the brain regions of SNAP25-150Q (150Q; line-2) mice at 2 mo of age. Anti-SNAP25 staining reveals that transgenic SNAP25-20Q is expressed at a higher level than SNAP25-150Q and that both SNAP25-20Q and SNAP25-150Q are lower than endogenous SNAP25. Anti-htt also labeled nonspecific bands above 50 kD in the liver and lung samples. Endog., endogenous.



