Fats are the major forms of energy storage for organisms. Fat storage is tightly associated with human health. The disruption of fat storage leads to severe human diseases including obesity, lipodystrophy, diabetes and fat liver. Congenital generalized lipodystrophy type 2 (CGL2), one the most severe lipodystrophy diseases in human, is caused bymutation of the Seipin gene.Seipin plays an important role in adipocytedifferentiation and lipid homeostasis, but its functions are stillunclear.
Dr. HUANG Xun and his colleagues at the Institute of Genetics and Developmental Biology, Chinese Academy of Sciencesgenerated a lipodystrophy model in Drosophila with a mutationin the Seipin gene. Their previous studies published on PLoS Genetics in 2011 suggest thatSeipin may tissue-autonomously participate in phosphatidic acid metabolism and subsequently prevent ectopiclipid droplet formation.
In a recent study, researchers from Xun Huang’s Group found that Seipin physically interacts with the sarco/endoplasmic reticulum Ca2+-ATPase (SERCA) in both Drosophila and man. SERCA, an endoplasmic reticulum (ER) calcium pump, is solely responsible for transporting cytosolic calcium into the ER lumen. Like Seipin, SERCA cell-autonomously promotes lipid storage in Drosophila fat cells. The ER lumen region of Seipin is necessary for the Seipin-dSERCA interaction and the self-oligomerization of Seipin. Seipin affects SERCA activity and modulates intracellular calcium homeostasis. Adipose tissue-specific knockdown of the ER-to-cytosol calcium release channel RyR partially restores fat storage in dSeipin mutants. Seipin may affect lipogenesis and fatty acid β-oxidation in fat cells. The resultsreveal thatSeipin promotesadipose tissue fat storage by regulating intracellularcalcium homeostasis.This finding may lead to an effective therapy for CGL2.
This work has been published on
Cell Metabolism(
http://dx.doi.org/10.1016/j.cmet.2014.03.028), with BI Junfeng as the first author.This research was supported by Ministry of Science and Technology of China, National Natural Science Foundation of China, and the Chinese Academy of Sciences.
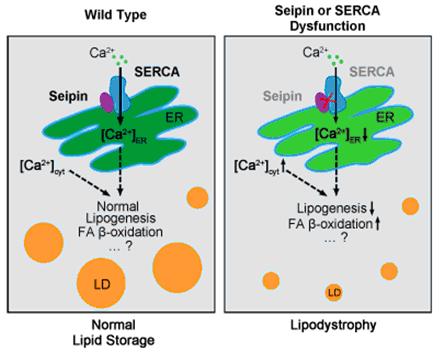
Figure.The working model of Seipin-SERCA mediated calcium homeostasis in fat storage regulation. (Image by IGDB)



