Protein post-translational modification by ubiquitination participates in many aspects of plant growth, development and stress responses. Covalent attachment of ubiquitin to targets is sequentially catalyzed by three enzymes (E1, E2 and E3). Abscisic acid (ABA) is a major phytohormone that affects plant growth and development as well as various biotic and abiotic stress responses. Recently, the regulatory role of ubiquitination in ABA signaling has been extensively studied, especially the subtle modulation of PYR/PYL/RCAR-type ABA receptors by two multi-units E3 ubiquitin ligase complexes and one single-unit E3 ubiquitin ligase through 26S proteasome system. Most studies focused on identification and function analysis of E3 ubiquitin ligase, but the function of E2 or E2-like in ABA signaling is poorly understood.
The team led by Prof. XIE Qi’s lab at the Institute of Genetics and Developmental Biology, Chinese Academy of Sciences, revealed how an E2-like protein VPS23A modulates ABA signaling, and a new mechanism of ABA receptors turnover.
In this study, the researchers found E2-like VPS23A is a key component of ESCRT-I, and negatively regulates ABA signaling. They found that VPS23A has epistatic relation with PYR/PYL/RCAR type ABA receptors. VPS23A may recognize both the unmodified PYR/PYLs and the K63-linked diubiquitin chains of PYR/PYLs. VPS23A affects the subcellular localization of PYR1 and stability of the PYL4 by the analysis of vps23a mutant.
These findings revealed that VPS23A affects PYR1/PYL4 via vacuole-mediated degradation besides 26S proteasome system. Moreover, these results make people understand more clearly both the turnover of ABA receptors and ESCRTs in plant hormone signaling.
This study entitled “ESCRT-I component VPS23A affects ABA signaling by recognizing ABA receptors for endosomal degradation” has been published online in
Mol. Plant (
DOI:10.1016/j.molp.2016.11.002).
This research is supported by the Key Project Grant of Ministry of Science and Technology of China and the National Natural Science Foundation of China.
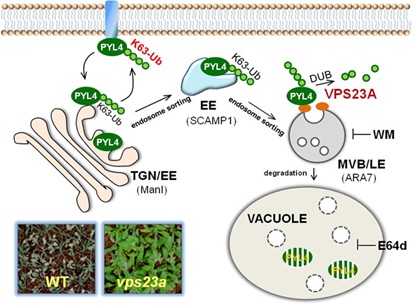
The turnover of PYLs ABA receptor by the endosomal trafficking non-26S proteasome degradation pathway. (Image by IGDB)
Contact:
Dr. XIE Qi
Email: qxie@genetics.ac.cn



